CALCIUM HYDRIDE
- CAS No.
- 7789-78-8
- Chemical Name:
- CALCIUM HYDRIDE
- Synonyms
- CaH2;Hydrolete;Hydrolith;cium hydride;Cakium hydride;CALCIUM HYDRIDE;Dihydridecalcium;Calciumhydridemm;Calciumhydride,95%;Calciumhydridemesh
- CBNumber:
- CB0115234
- Molecular Formula:
- CaH2
Lewis structure
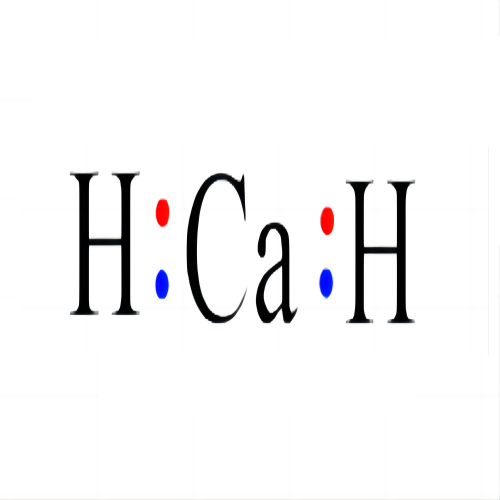
- Molecular Weight:
- 42.09
- MDL Number:
- MFCD00010897
- MOL File:
- 7789-78-8.mol
| Melting point | 816 °C (lit.) |
|---|---|
| Density | 1.9 |
| storage temp. | Store below +30°C. |
| solubility | reacts with H2O, ethanol |
| form | powder |
| color | Light gray |
| Specific Gravity | 1.9 |
| Water Solubility | Soluble in water and alcohol. Insoluble in benzene. |
| Sensitive | Moisture Sensitive |
| Merck | 14,1672 |
| Stability | Stable, but reacts violently with water, liberating and igniting hydrogen. Contact with strong oxidizers may cause fire or explosion. Incompatible with strong oxidizing agents, strong acids, halogens, water, alcohols. |
| InChIKey | FAQLAUHZSGTTLN-UHFFFAOYSA-N |
| CAS DataBase Reference | 7789-78-8(CAS DataBase Reference) |
| FDA UNII | WY779SQ0XW |
| NIST Chemistry Reference | calcium hydride(7789-78-8) |
| EPA Substance Registry System | Calcium hydride (CaH2) (7789-78-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H260-H315-H319 | |||||||||
| Precautionary statements | P223-P231+P232-P264-P280-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | F | |||||||||
| Risk Statements | 15 | |||||||||
| Safety Statements | 24/25-43-7/8-43A | |||||||||
| RIDADR | UN 1404 4.3/PG 1 | |||||||||
| WGK Germany | 1 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 4.3 | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 28500090 | |||||||||
| NFPA 704 |
|
CALCIUM HYDRIDE price More Price(35)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 21170 | Calcium hydride purum p.a., ≥97.0% (gas-volumetric), powder | 7789-78-8 | 25g | $114 | 2024-03-01 | Buy |
| Sigma-Aldrich | 21170 | Calcium hydride purum p.a., ≥97.0% (gas-volumetric), powder | 7789-78-8 | 100g | $325 | 2024-03-01 | Buy |
| Sigma-Aldrich | 21170 | Calcium hydride purum p.a., ≥97.0% (gas-volumetric), powder | 7789-78-8 | 500g | $1130 | 2024-03-01 | Buy |
| Sigma-Aldrich | 208027 | Calcium hydride reagent grade, 95% (gas-volumetric) | 7789-78-8 | 5g | $55 | 2024-03-01 | Buy |
| Sigma-Aldrich | 208027 | Calcium hydride reagent grade, 95% (gas-volumetric) | 7789-78-8 | 100g | $178 | 2024-03-01 | Buy |
CALCIUM HYDRIDE Chemical Properties,Uses,Production
Alkaline earth metal
Calcium hydride is an alkaline earth metal hydride, although it is not stable than lithium hydride, but stable than other alkali metal borohydride. Chemical formula is CaH2. Molecular weight is 42.10. It is white monoclinic crystals or lumps. Industrial is gray. When exposed to moist air hydrogen will release, and calcium hydroxide will leave. The proportion of is 1.9. The decomposition tempreture is about 600℃. Melting point is 816℃ (hydrogen). When meet water, carboxylic acids, lower alcohols, it can decompose to generate hydrogen. The decomposition When tempreture get 600℃, it begins to decompose. At room temperature, it can not react with dry oxygen, nitrogen, chlorine, but can react at high temperatures. This reaction can generate CaO, Ca3N2, CaCl2, respectively. At room temperature, it can react with water and the product is calcium hydroxide and hydrogen.
Calcium hydride has a strong reduction, and can make the metal liberate from the metal oxides, metal chlorides, for example,
2CaH2 + MO2→ 2CaO+2H2 + M (metal).
Calcium hydride can be used for strong reducing agent commonly, as well as used to make hydrogen when work in the field.
Preparation: The calcium metal is charged in the wok, and it can react with hydrogen to generate calcium hydride when temperature get about 300℃ with electric or oil.
Ca + H2 → CaH2 + 214kJ·mol-1
Or in a stream of hydrogen, magnesium can reduce calcium oxide reduction to get calcium hydride, due to the separation of calcium oxide is difficult, high purity calcium hydride products can not obtain.
CaO + Mg + H2 → CaH2 + MgO
Purpose: When be heated to 600 ~1000 ℃, the oxide of zirconium, niobium, uranium and chromium can be reduced to prepare powder of these metals, so calcium hydride can be used in powder metallurgy. It can be insoluble in ether, can react with ethanol to produce hydrogen and ethanol calcium.
Hydrogen can be obtained by the reaction of water, one gram of the product in the water can release one liter of hydrogen, so it is often used as a portable source of hydrogen. In addition, calcium hydride is also used dehydration of organic compounds, hydrogenation, condensation agent; or as a drying agent, the drying effect is better than sodium, phosphorus pentoxide.
The above information is edited by the chemicalbook of Wang Xiaodong.
Toxicity
When meet moisture, water or acids, hydrogen can be released and can cause combustion, it can react with oxidants and metal oxides violent. Dust can cause strong stimulating effect for the eyes, nose, skin and respiratory system. The product of calcium hydroxide is strong corrosive when meet moisture.
Other reference lithium hydride.
Chemical Properties
It is colorless orthorhombic crystallization; gray goods, orthorhombic or powder. It is sensitive to moisture. The relative density is 1.90. Melting point is 816℃ (in hydrogen). It can decompose to Ca and H2 at 600℃. With water it can decompose and release hydrogen at the same time, it can also generate hydrogen and ethanol calcium when react with ethanol. The reduction of metal oxides is more strongly than sodium hydride or lithium hydride.
Uses
It can be used as reducing agent and a condensing agent in organic synthesis, and desiccant in producing hydrogen material.
Production method
The purity of about 99.5% purified calcium is put into the iron plate, then put on the central quartz reaction tube, at both ends of the quartz reaction tubes are installed into the trachea and a tube with rubber stopper, the purification of hydrogen go through from the intake pipe, the trachea is contact with by mineral oil bubbler and fume hood. The reaction tubes is electric heating.
At beginning, raction air in the system is replaced by large purified hydrogen, then heated by an electric furnace. The reaction starts from about 200℃, further heated to 250~300℃, then a flow rate of 0.6 m 1/ min of hydrogen gas introduce into the reaction, the reaction needs about 2h to complete, the product of calcium hydride is porous white crystalline powder, the purity of calcium hydride is about 99%.
Ca + H2 → CaH2
Category
Explosive substances.
Explosive hazardous characteristics
When the reaction was heated with tetrahydrofuran, it can cause explosion; when mix with potassium chlorate, hypochlorite, bromate, perchlorate, it is heat sensitive, friction sensitive and explosive.
Storage Characteristics
Treasury need ventilation low temperature drying; shockproof, moistureproof, against high temperature.
Extinguishing agent
Foam, carbon dioxide, dry powder.
Description
Calcium hydride is a gray powder (white if pure,
which is rare). It reacts vigorously with water liberating
H2 gas. CaH2 is thus used as a drying agent,
i.e. a desiccant. It is prepared directly from the metal
or by reacting CaCO3 with hydrogen at elevated temperatures. The overall reaction is shown as
follows:
CaCO3+heat+H2→CaH2+H2O+CO2
CaH2 is a saline hydride, meaning that its structure is
salt-like. The alkali metals and the alkaline earth metals
all form saline hydrides. These species are insoluble in
all solvents with which they do not react because they
have extended structures. CaH2 crystallizes in the
PbCl2 structural pattern.
The reaction of CaH2 with water can be represented
as follows:
CaH2+2H2O0Ca(OH)2+2H2
The two hydrolysis products, H2, a gas, and Ca(OH)2,
an aqueous mixture of solid plus liquid (i.e. a slurry), are readily separated from the solvent by distillation, filtration, or decantation.
Chemical Properties
Pure calcium hydride crystallizes in the form of colorless, hexagonal prisms, but the industrial product contains some calcium metal and is consequently gray. On heating, calcium hydride decomposes without melting. In keeping with its salt-like character it is insoluble in inert solvents but dissolves in molten LiCl – KCl eutectic mixtures (352℃). Calcium hydride can be safely handled in air, but is slowly attacked and crumbles to powder if the air is moist. It reacts with dry oxygen or nitrogen if heated above 500℃, forming the oxide or nitride, respectively.
Physical properties
Grayish orthorhombic crystal or powder; stable at ambient temperature; density 1.70 g/cm3; melts at 816°C; reacts with water and alcohol.
Uses
Calcium hydride is used as an efficient drying agent for aprotic base-stable solvents like ethers and tertiary amines. It is a useful dehydrating agent in a synthesis of aldehyde enamines in high yield and purity.
Uses
Calcium hydride is a relatively mild desiccant. It is safer than the more reactive agents such as sodium metal. Calcium hydride is widely used as a desiccant for basic solvents such as amines and pyridine in organic syntheses. It is also used to pre-dry solvents prior to the use of a more reactive desiccant. The compound has, however, been widely used for decades as a safe and convenient means to inflate weather balloons. Likewise, it is regularly used in laboratories to produce small quantities of highly pure hydrogen for experiments.
Uses
To prepare rare metals by reduction of their oxides; as a drying agent for liquids and gases; to generate hydrogen: 1 g of calcium hydride in water liberates 1 liter of hydrogen at STP; in organic syntheses.
Preparation
Calcium hydride may be prepared from its elements by direct combination of calcium and hydrogen at 300 to 400°C. It also can be made by heating calcium chloride with hydrogen in the presence of sodium metal:
CaCl2 + H2 + 2Na → CaH2 + NaCl
Alternatively, calcium hydride may be prepared by the reduction of calcium oxide with magnesium in the presence of hydrogen:
CaO + Mg + H2 → CaH2 + MgO.
Production Methods
Calcium hydride ignites in air on heating and can explode violently if mixed and rubbed with a strong oxidizing agent such as perchlorate or bromate. Contact with water produces hydrogen which can create a fire hazard in a confined space.
Reactions
Once
ignited, it burns with a strongly exothermic reaction:
CaH2+O2→CaO+H2O
The reaction of calcium hydride with water is
also very exothermic. Heating is necessary to
initiate the reaction with alcohols to form alcoholates. The reaction with ammonia takes place
at 700 ℃ with formation of calcium amide. At
high temperature (600 – 1000℃) calcium
hydride is a powerful reducing agent.
General Description
Grayish-colored lump or crystalline solid. Irritating to skin and eyes. Used to make other chemicals.
Air & Water Reactions
Ignites in air or reacts violently, sometimes explosively, with air of high humidity [Bretherick 1979 p. 107]. Reacts exothermically with water to generate flammable hydrogen gas and calcium hydroxide, a base. [Merck, 11th ed. 1989].
Reactivity Profile
When silver fluoride is ground with CALCIUM HYDRIDE the mass becomes incandescent [Mellor 3:389 1946-47]. Heating the hydride strongly with chlorine, bromine, or iodine leads to incandescence. Mixtures of the hydride with various bromates, i.e. barium bromate; chlorates, i.e. barium chlorate, and perchlorates, i.e. potassium perchlorate; explode on grinding, [Mellor, 1946, vol. 3, 651]. CaH2 reacts incandescently with AgF if subject to friction. (Mellor, 1941, Vol. 3, 389, 651).
Hazard
Evolves highly flammable hydrogen when wet; solid product is slaked lime. Irritating to skin.
Health Hazard
Inhalation or contact with vapors, substance or decomposition products may cause severe injury or death. May produce corrosive solutions on contact with water. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.
Fire Hazard
Produce flammable gases on contact with water. May ignite on contact with water or moist air. Some react vigorously or explosively on contact with water. May be ignited by heat, sparks or flames. May re-ignite after fire is extinguished. Some are transported in highly flammable liquids. Runoff may create fire or explosion hazard.
Flammability and Explosibility
Not classified
Potential Exposure
Calcium hydride is used as a dryingand reducing agent and a cleaner for blocked up oil wells.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for atleast 30 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure, beginrescue breathing (using universal precautions, includingresuscitation mask) if breathing has stopped and CPR if heartaction has stopped. Transfer promptly to a medical facility.When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Donot make an unconscious person vomit. Medical observationis recommended for 24-48 h after breathing overexposure,as pulmonary edema may be delayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consideradministering a corticosteroid spray.
storage
Color Code—Red Stripe: Flammability Hazard:Do not store in the same area as other flammable materials.Calcium hydride must be stored to avoid contact with wateror steam since violent reactions occur and flammablehydrogen gas is produced. Store in tightly closed containersin a cool, well-ventilated area.
Shipping
Calcium hydride should carry a “DANGEROUSWHEN WET” label. It falls in Hazard Class 4.3 andPacking Group I.
Incompatibilities
Reacts with water, moist air, and steam,releasing flammable hydrogen gas and may self-ignite inair. Incompatible with metal halogenates, silver fluoride,and tetrahydrofuran.
CALCIUM HYDRIDE Preparation Products And Raw materials
Raw materials
Preparation Products
1of3
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Firsky International Trade (Wuhan) Co., Ltd | +8615387054039 | admin@firsky-cn.com | China | 436 | 58 |
| Hangzhou ICH Biofarm Co., Ltd | +86-0571-28186870; +undefined8613073685410 | sales@ichemie.com | China | 990 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21666 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29888 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8820 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shanghai Longyu Biotechnology Co., Ltd. | +8619521488211 | info@longyupharma.com | China | 2534 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49392 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12446 | 58 |
View Lastest Price from CALCIUM HYDRIDE manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-11-01 | Calcium Hydride Low Nitrogen
7789-78-8
|
US $0.00 / kg | 200kg | 99% | plenty of stock | Hangzhou ICH Biofarm Co., Ltd | |
 |
2023-10-08 | CALCIUM HYDRIDE
7789-78-8
|
US $30.00 / KG | 1KG | 99% | 20T | Firsky International Trade (Wuhan) Co., Ltd | |
 |
2023-06-29 | CALCIUM HYDRIDE
7789-78-8
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd |
-
- Calcium Hydride Low Nitrogen
7789-78-8
- US $0.00 / kg
- 99%
- Hangzhou ICH Biofarm Co., Ltd
-

- CALCIUM HYDRIDE
7789-78-8
- US $30.00 / KG
- 99%
- Firsky International Trade (Wuhan) Co., Ltd
-

- CALCIUM HYDRIDE
7789-78-8
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
7789-78-8(CALCIUM HYDRIDE)Related Search:
1of4





