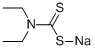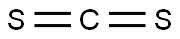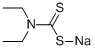Sodium diethyldithiocarbamate
- CAS No.
- 148-18-5
- Chemical Name:
- Sodium diethyldithiocarbamate
- Synonyms
- DDTC;DTC;dithiocarbamate;dedc;NADEDC;Sodium N,N-diethyldithiocarbamate;Sodium Diethyldithiocarbamate 97 %;DIETHYLDITHIOCARBAMIC ACID SODIUM SALT;SDEC;dedk
- CBNumber:
- CB0232773
- Molecular Formula:
- C5H10NNaS2
- Molecular Weight:
- 171.26
- MDL Number:
- MFCD00041028
- MOL File:
- 148-18-5.mol
- MSDS File:
- SDS
| Melting point | 95°C |
|---|---|
| Density | 1.1000 |
| vapor pressure | 0Pa at 20℃ |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | about 35 % in water, less soluble in organic solvents. However, the free acid, diethyldithiocarbamic acid, is readily soluble in organic solvents and less soluble in water. Thus, on acidification of an aqueous solution of a diethyldithiocarbamate, the free acid can be extracted with chloroform or carbon tetrachloride. The distribution ratio is 2,360 for chloroform and 343 for carbon tetrachloride. |
| form | Liquid |
| Specific Gravity | 1.08 |
| color | Clear, colorless |
| Odor | wh., sl. brown or sl. pink odorless cryst. |
| Water Solubility | >=10 g/100 mL at 14 ºC |
| Hydrolytic Sensitivity | 0: forms stable aqueous solutions |
| LogP | -1.1 at 20℃ |
| CAS DataBase Reference | 148-18-5(CAS DataBase Reference) |
| FDA UNII | A5304YEB5E |
| NCI Drug Dictionary | ditiocarb sodium |
| IARC | 3 (Vol. 12, Sup 7) 1987 |
| EPA Substance Registry System | Sodium diethyldithiocarbamate (148-18-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS09,GHS07,GHS05 |
|---|---|
| Signal word | Danger |
| Hazard statements | H410-H302-H314 |
| Precautionary statements | P273-P391-P501-P260-P264-P280-P301+P330+P331-P303+P361+P353-P363-P304+P340-P310-P321-P305+P351+P338-P405-P501-P264-P270-P301+P312-P330-P501 |
| RIDADR | 3077 |
| HazardClass | 9 |
| PackingGroup | III |
Sodium diethyldithiocarbamate price More Price(17)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Usbiological | 289468 | Sodium N- | 148-18-5 | 1g | $333 | 2021-12-16 | Buy |
| Usbiological | 466302 | Sodium N- | 148-18-5 | 5mg | $425 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FD38054 | Ditiocarb sodium - 40%, in water | 148-18-5 | 10g | $150 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FD38054 | Ditiocarb sodium - 40%, in water | 148-18-5 | 25g | $300 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FD38054 | Ditiocarb sodium - 40%, in water | 148-18-5 | 50g | $500 | 2021-12-16 | Buy |
Sodium diethyldithiocarbamate Chemical Properties,Uses,Production
Chemical Properties
Sodium diethyldithiocarbamate is a white or colorless crystalline powder, easily soluble in water and alkaline, soluble in alcohol, rapidly decomposed in acidic aqueous solution to separate out carbon disulfide and make the solution cloudy. In ammonia medium, it generates precipitate or brown colloidal solution with copper ion. Copper reagent is applied in the color development reaction of copper, used as precipitant and solvent extractant of soft metal ions, also used as photometric reagent for the determination of bismuth, copper, nickel and other metals.
Uses
Sodium diethyldithiocarbamate is an organic molecular entity. It is an inhibitor of superoxide dismutase, having both antioxidant and oxidant effects.
Uses
Sodium diethyldithiocarbamate reacts with even more metal ions than does dithizone. However, its analytical application is seriously limited by its considerably lower stability in acidic aqueous media. According to the examinations of Bode,the reagent undergoes considerable decomposition within 5 minutes at pH 5. The analytical application of the reagent is therefore restricted to a very narrow pH range. There is no chance with this reagent to enhance the analytical selectivity of extractions by making use of the difference between the stabilities of its complexes with various metal ions and to choose the pH of the reaction mixture accordingly.
Preparation
This salt is obtained by treating carbon disulfide with diethylamine in the presence of sodium hydroxide:
CS2+ HN(C2H5)2+ NaOH → NaS2CN(C2H5)2+ H2O
Definition
ChEBI: Sodium diethyldithiocarbamate is an organic molecular entity.
General Description
Odorless white or slightly brown or slightly pink crystals.
Air & Water Reactions
Water soluble. Thio and dithiocarbamates slowly decompose in aqueous solution to form carbon disulfide and methylamine or other amines. Such decompositions are accelerated by acids.
Reactivity Profile
Sodium diethyldithiocarbamate is not compatible with strong oxidizing agents. Aqueous solutions slowly decompose to form carbon disulfide and an amine. Such decompositions are accelerated by acids. Addition of acid to the aqueous solution produces a white turbidity .
Fire Hazard
Flash point data for Sodium diethyldithiocarbamate are not available; however, Sodium diethyldithiocarbamate is probably combustible.
Flammability and Explosibility
Non flammable
Safety Profile
Moderately toxic by ingestion, intraperitoneal, and subcutaneous routes. Experimental reproductive effects. Questionable carcinogen with experimental neoplastigenic and teratogenic data. Human mutation data reported. When heated to decomposition it emits very toxic fumes of NOx, SOx, and Na2O. Used as a pesticide. See also CARBAMATES
Sodium diethyldithiocarbamate Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 3002 | 58 |
| Qingdao RENAS Polymer Material Co., Ltd. | +86-0532-86867058 +86-18562606086 | sales@qdrenas.com | China | 316 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29798 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21666 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9294 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29888 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5909 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39916 | 58 |
| Shenyang Simchoice Chemical Co.,Ltd | +86-024-23769576 +86-15040101888 | sales@simchoicechem.com | China | 255 | 58 |
View Lastest Price from Sodium diethyldithiocarbamate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-09-13 | Sodium dibutyl dithiocarbamate
148-18-5
|
US $0.00 / kg | 20kg | 90 | 20 tons | Qingdao RENAS Polymer Material Co., Ltd. | |
 |
2024-05-16 | Sodium diethyldithiocarbamate
148-18-5
|
US $36.00 / kg | 1kg | 99% | 5000kg/week | Hebei Zhuanglai Chemical Trading Co.,Ltd | |
 |
2022-09-12 | Sodium diethyldithiocarbamate
148-18-5
|
US $0.00-0.00 / KG | 1KG | 98% | 1ton | Henan Aochuang Chemical Co.,Ltd. |
-

- Sodium dibutyl dithiocarbamate
148-18-5
- US $0.00 / kg
- 90
- Qingdao RENAS Polymer Material Co., Ltd.
-

- Sodium diethyldithiocarbamate
148-18-5
- US $36.00 / kg
- 99%
- Hebei Zhuanglai Chemical Trading Co.,Ltd
-

- Sodium diethyldithiocarbamate
148-18-5
- US $0.00-0.00 / KG
- 98%
- Henan Aochuang Chemical Co.,Ltd.