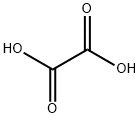
Oxalic acid
- CAS No.
- 144-62-7
- Chemical Name:
- Oxalic acid
- Synonyms
- oxalic;caosuan;ETHANEDIOIC ACID;HOOCCOOH;kyselinastavelova;DI-CARBOXYLIC ACID;Oxalic acid anhydrous;Oxalsαure;Oxalsaeure;xalic Acid
- CBNumber:
- CB0323998
- Molecular Formula:
- C2H2O4
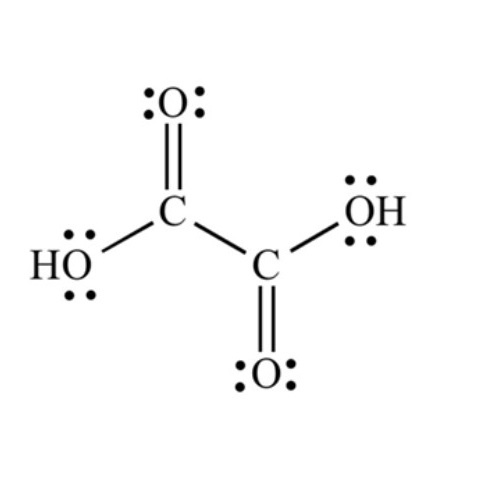
Lewis structure
- Molecular Weight:
- 90.03
- MDL Number:
- MFCD00002573
- MOL File:
- 144-62-7.mol
- MSDS File:
- SDS
| Melting point | 189.5 °C (dec.)(lit.) |
|---|---|
| Boiling point | 365.1°C (estimate) |
| Density | 0.99 g/mL at 25 °C |
| vapor density | 4.4 (vs air) |
| vapor pressure | <0.01 mm Hg ( 20 °C) |
| refractive index | 1.4261 (estimate) |
| Flash point | 101-157°C |
| storage temp. | Store below +30°C. |
| solubility | water: soluble108g/L at 25°C |
| form | Liquid |
| pka | 1.23(at 25℃) |
| color | White |
| PH Range | 6 - 8 at 25 °C |
| PH | 3(1 mM solution);2.09(10 mM solution);1.31(100 mM solution); |
| Odor | odorless |
| Water Solubility | 90 g/L (20 ºC) |
| Sublimation | 101-157 ºC |
| Merck | 14,6911 |
| BRN | 385686 |
| Henry's Law Constant | 1.43 at pH 4 (quoted, Gaffney et al., 1987) |
| Exposure limits | NIOSH REL: TWA 1, STEL 2, IDLH 500; OSHA PEL: TWA 1; ACGIH TLV: TWA 1, STEL 2 (adopted). |
| Stability | Stable, but moisture sensitive. Incompatible with metals. |
| InChIKey | MUBZPKHOEPUJKR-UHFFFAOYSA-N |
| LogP | -1.7 at 23℃ |
| Indirect Additives used in Food Contact Substances | OXALIC ACID |
| FDA 21 CFR | 177.2410 |
| CAS DataBase Reference | 144-62-7(CAS DataBase Reference) |
| EWG's Food Scores | 2-4 |
| FDA UNII | 9E7R5L6H31 |
| NIST Chemistry Reference | Oxalic acid(144-62-7) |
| EPA Substance Registry System | Oxalic acid (144-62-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302+H312-H318 | |||||||||
| Precautionary statements | P264-P270-P280-P301+P312-P302+P352+P312-P305+P351+P338 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 21/22-63-34-41 | |||||||||
| Safety Statements | 24/25-23-36/37/39-27-26-39-37-36-36/37 | |||||||||
| OEB | C | |||||||||
| OEL | TWA: 1 mg/m3, STEL: 2 mg/m3 | |||||||||
| RIDADR | UN 3261 8/PG 3 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | RO2450000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29171110 | |||||||||
| Toxicity | LD50 orally in Rabbit: 375 mg/kg | |||||||||
| IDLA | 500 mg/m3 | |||||||||
| NFPA 704 |
|
Oxalic acid price More Price(50)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.16144 | Oxalic acid anhydrous for synthesis | 144-62-7 | 50g | $62.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.16144 | Oxalic acid anhydrous for synthesis | 144-62-7 | 250g | $197 | 2024-03-01 | Buy |
| Sigma-Aldrich | 658537 | Oxalic acid purified grade, 99.999% trace metals basis | 144-62-7 | 1000G | $2190 | 2024-03-01 | Buy |
| Sigma-Aldrich | 194131 | Oxalic acid 98% | 144-62-7 | 5g | $48.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 194131 | Oxalic acid 98% | 144-62-7 | 1kg | $176 | 2024-03-01 | Buy |
Oxalic acid Chemical Properties,Uses,Production
description
Oxalic acid is a strong dicarboxylic acid occurring in many plants and vegetables, usually as its calcium or potassium salts. Oxalic acid is the only possible compound in which two carboxyl groups are joined directly; for this reason oxalic acid is one of the strongest organic acids. Unlike other carboxylic acids (except formic acid), it is readily oxidized; this makes it useful as a reducing agent for photography, bleaching, and ink removal. Oxalic acid is usually prepared by heating sodium formate with sodium hydroxide to form sodium oxalate, which is converted to calcium oxalate and treated with sulfuric acid to obtain free oxalic acid.
concentrations of oxalic acid are pretty low in most plants and plant-based foods, but there’s enough in spinach, chard and beet greens to interfere with the absorption of the calcium these plants also contain.
It is produced in the body by metabolism of glyoxylic acid or ascorbic acid. It is not metabolized but excreted in the urine. It is used as an analytical reagent and general reducing agent.Oxalic acid is a natural acaricide used for treatment against varroa mites in colonies with no/low brood, packages, or swarms. Vaporized oxalic acid is used by some beekeepers as an insecticide against the parasitic Varroa mite.
Chemical properties
Oxalic acid is widely distributed in the plant in nature, most existing in the form of oxalic acid salt. C.W. Scheele had for the first time manufactured oxalate in 1776.
Oxalate is the strongest acid among the dicarboxylic acid. Besides having the general properties of the carboxylic acid, it also has reducing property and can quantitatively reduce the seven valence manganese to bivalent manganese. This property is often used for quantitative analysis of potassium permanganate.
5 C2H2O4 + 2 KMnO4 + 3 H2SO4 →K2SO4 + 2 MNSO4 + 8H2O + 10 CO2;
Oxalic acid can also reduce the trivalent iron into bivalent iron. Because of the high solubility of the bivalent iron in the water, we can apply this principle to remove rust on the clothes.
Oxalic acid can react with phosphorus pentachloride to generate phosphorus oxychloride. C2H2O4 + PCl5 → POCl3 + CO + CO2 + 2 HCL.
Oxalic acid can react with many metals to produce oxalic acid salt. In addition to the alkali metal salt and bivalent iron salts with the rest of the oxalic acid salt being poorly soluble in water. Some metal salt, although is poorly soluble in water, can generate complex that is soluble in water.
Fe2 (C2O4) 3 + 3 K2C2O4 + 6 H2O →2 K3 [Fe (C2O4) 3] • 6 H2O.
Upon heating, alkali metal and alkaline earth metal oxalic acid salt can lose carbon monoxide and form carbonates with carbonate continuing to be subject to heating to be further decomposed into oxide and carbon dioxide. The oxalic acid salt of nickel, cobalt and silver can finally produce metal instead of nonmetal oxide.
The decomposing products of the oxalate are carbon dioxide, carbon monoxide and water.
Oxalate and oxalic acid salt are toxic. Mice, through oral administration, has LD50 of 2000~4000 mg/kg.
The above information is edited by the chemicalbook of Dai Xiongfeng.
Uses
1. Oxalic acid can be mainly used as reducing agent and bleaching agent, mordant for dyeing and printing industry, also used in refining rare metal, the synthesis of various oxalate ester amide, oxalate and grass, etc.
2. Used as analytical reagent.
3. Used as laboratory reagents, chromatography analysis reagent, dye intermediates and standard material.
4. Oxalic acid is mainly used for producing drugs such as antibiotics and borneol and solvent for extracting the rare metal, reducing agent and dye, tanning agent, etc. In addition, oxalic acid can also be used for the synthesis of various kinds of oxalate ester, oxalate, and oxamide with diethyl oxalate, sodium oxalate and calcium oxalate having the largest yield. Oxalate can also be used for the production of cobalt-molybdenum-alumina catalyst, cleaning of metal and marble as well as the bleaching of textiles.
First aid
This kind of chemical, in case of contact with eyes and skin, should be quickly rinsed with plenty of water; Upon inhaling of large amount, we should immediately move the victim away from the scene to fresh air place; if necessary, apply artificial respiration; if be swallowed, immediately give medical injection with plenty of water for rapid gastric lavage and apply symptomatic treatment.
Protective measures
Upon productive operation, we must wear protective work cloth in order to prevent the skin repeatedly or long-term contact. Wear protective glasses to prevent eye contact. For the nitrogen oxide gas produced during the production process, measures should be taken to absorb before discharge. Work clothes if contaminated, should be replaced every day. Permeable work clothes, if getting wet, should be taken off quickly.
Medical Surveillance
During employment and regular physical examination, we should check the skin, respiratory tract, and kidney function. If there is growing oxalic acid salt crystal in the urine, it is helpful to ascertain the oral poisoning. We should pay attention to follow-up. The determination of blood calcium and blood oxalic acid salt content is also suitable for this purpose.
Storage
We should hold it with glass bottle, cask, multi-layer paper bag or metal barrel to prevent mechanical damage. It should be placed in cool, ventilated, dry place for sealed storage. It is best to use the open warehouse and should be placed far away from any places that have potential risk of serious fire. It should be stored separately from antioxidant.
waste disposal
Pre-processing includes chemical reaction with limestone or calcium oxide to generate calcium oxalate. It can be then subject to calcination and can put into particle collection device to collect for reuse.
Description
Oxalic acid is a colorless, odorless powder orgranular solid. The anhydrous form (COOH)2 is an odorless,white solid; the solution is a colorless liquid. Molecularweight = 90.04; Specific gravity (H2O:1) = 1.90; Sublimationpoint =150-157℃; Freezing/Melting point (decomposes):101.7℃; 190℃ (anhydrous). Hazard Identification (based onNFPA-704 M Rating System): Health 3, Flammability 1,Reactivity 0. Moderately soluble in water.
Chemical Properties
Oxalic acid is a colorless, odorless powder, or granular solid. The anhydrous form (COOH)2 is an odorless, white solid; the solution is a colorless liquid.
Physical properties
Colorless and odorless rhombic crystals. Hygroscopic. soluble in ethanol, soluble in water, slightly soluble in ether, insoluble in benzene and chloroform.
Uses
Oxalic acid was used: · in the synthesis of hemicellulose hydrolysates of yellow poplars; · in the synthesis of three-dimensionally ordered macroporous metal oxides or carbonates via templating with polystyrene spheres; · as supporting electrolyte in the electrochemical synthesis of polyaniline-polypyrrole composite coatings.
Uses
An impurity of oxaliplatin which is a coordination complex that is used in cancer chemotherapy. A reducing agent and its conjugate base, known as oxalate (C2O42?), is a useful chelating agent for metal cations.
Uses
Oxalic acid is made by the action of nitric acid on sugars, starch, or cellulose. This highly poisonous colorless crystal is soluble in water, alcohol, and ether. It was used to make ferric oxalate, as a preservative for pyrogallic acid developers, as a sensitizer for platinum papers, and to reduce the density of cyanotype prints.
Production Methods
Many industrial processes have been employed for the manufacture of oxalic acid since it was first synthesized. The following processes are in use worldwide: oxidation of carbohydrates, the ethylene glycol process, the propylene process, the dialkyl oxalate process, and the sodium formate process. Sodium formate process is no longer economical in the leading industrial countries, except for China.
Nitric acid oxidation is used where carbohydrates, ethylene glycol, and propylene are the starting materials. The dialkyl oxalate process is the newest, where dialkyl oxalate is synthesized from carbon monoxide and alcohol, then hydrolyzed to oxalic acid. This process has been developed by UBE Industries in Japan.Many attempts have been made to synthesize oxalic acid by electrochemical reduction of carbon dioxide in either aqueous or nonaqueous electrolytes.
Definition
ChEBI: Oxalic acid is an alpha,omega-dicarboxylic acid that is ethane substituted by carboxyl groups at positions 1 and 2. It has a role as a human metabolite, a plant metabolite and an algal metabolite. It is a conjugate acid of an oxalate(1-) and an oxalate.
Reactions
The reactions of oxalic acid, including the formation of normal and acid salts and esters, are typical of the dicarboxylic acids class. Oxalic acid, however, does not form an anhydride.
On rapid heating, oxalic acid decomposes to formic acid, carbon monoxide, carbon dioxide, and water. In aqueous solution, it is decomposed by uv, x-ray, or γ -radiation with the liberation of carbon dioxide. Photodecomposition also occurs in the presence of uranyl salts.
Oxalic acid is a mild reducing agent, and is oxidized by potassium permanganate in acid solution to give carbon dioxide and water. Oxalic acid is catalytically reduced by hydrogen in the presence of ruthenium catalyst to ethylene glycol, and electronically reduced to glyoxylic acid.
Oxalic acid reacts with various metals to form metal salts, which are quite important as the derivatives of oxalic acid. It also reacts easily with alcohols to give esters.
General Description
Odorless white solid. Sinks and mixes with water.
Air & Water Reactions
Water soluble. Hygroscopic
Reactivity Profile
Oxalic acid is hygroscopic and sensitive to heat. Oxalic acid may react violently with furfuryl alcohol, silver, sodium, perchlorate, sodium hypochlorite, strong oxidizers, sodium chlorite, acid chlorides, metals and alkali metals. . The heating of mixtures of Oxalic acid and urea has lead to explosions. This is due to the rapid generation of the gases, CO2, CO, and NH3, [Praxis Naturwiss. Chem., 1987, 36(8), 41-42]. Oxalic acid and urea react at high temperatures to form toxic and flammable ammonia and carbon monoxide gasses, and inert CO2 gas [Von Bentzinger, R. et al., Praxis Naturwiss. Chem., 1987, 36(8), 41-42].
Health Hazard
As dust or as a solution, can cause severe burns of eyes, skin, or mucous membranes. Ingestion of 5 grams has caused death with symptoms of nausea, shock, collapse, and convulsions coming on rapidly. Repeated or prolonged skin exposure can cause dermatitis and slow-healing ulcers.
Fire Hazard
Special Hazards of Combustion Products: Generates poisonous gases
Flammability and Explosibility
Non flammable
Agricultural Uses
Oxalic acid, (COOH)2, also called ethanedioic acid, is a
white, crystalline solid, slightly soluble in water. It is a
naturally occurring highly oxidized organic compound with significant chelating activity. It is strongly acidic
and poisonous, produced by many plants like sorrel
(sourwood), the leaf blades of rhubarb, bark of
eucalyptus and many plant roots. In plant cells and
tissues, oxalic acid gets accumulated as either sodium,
potassium or calcium oxalate, of which the latter occurs
as crystals. In turn, salts of oxalic acids enter the bodies
of animals and human beings, causing pathological
disorders, depending upon the amount consumed. Many
species of fungi like Aspergillus, Penicillium, Mucor, as
well as some lichens and slime moulds produce calcium
oxalate crystals. Upon the death of these microorganisms,
plants and animals, the salts get released into
the soil, causing some amount of toxicity. However,
oxalate-degrading microbes, called Oxalobacter
formigenes, decrease oxalate absorption in animals and
humans.
Oxalic acid is the first of a series of dicarboxylic
acids. It is used (a) as a bleaching agent for stains like rust
or ink, (b) in textile and leather production, and (c) as
monoglyceryl oxalate in the production of ally1 alcohol
and formic acid.
Safety Profile
Poison by subcutaneous route. Moderately toxic by ingestion. A skin and severe eye irritant. Acute oxalic poisoning results from ingestion of a solution of the acid. There is marked corrosion of the mouth, esophagus, and stomach, with symptoms of vomiting, burning abdominal pain, collapse, and sometimes convulsions. Death may follow quickly. The systemic effects are attributed to the removal by the oxalic acid of the calcium in the blood. The renal tubules become obstructed by the insoluble calcium oxalate, and there is profound hdney dlsturbance. The chief effects of inhalation of the dusts or vapor are severe irritation of the eyes and upper respiratory tract, gastrointestinal disturbances, albuminuria, gradual loss of weight, increasing weakness and nervous system complaints, ulceration of the mucous membranes of the nose and throat, epistaxis, headache, irritation, and nervousness. Oxalic acid has a caustic action on the skin and may cause dermatitis; a case of early gangrene of the fingers resembling that caused by phenol has been described. More severe cases may show albuminuria, chronic cough, vomiting, pain in the back, and gradual emaciation and weakness. The skin lesions are characterized by crachng and fissuring of the skin and the development of slow-healing ulcers. The skin may be bluish in color, and the nails brittle and yellow. Violent reaction with furfuryl alcohol, Ag, NaClO3, NaOCl. When heated to decomposition it emits acrid smoke and irritating fumes. See also OXALATES
Potential Exposure
Oxalic acid is used in textile finishing, paint stripping; metal and equipment cleaning; as an intermediate; as an analytic reagent and in the manufacture of dyes, inks, bleaches, and paint removers; varnishes, wood, and metal cleansers; dextrin, cream of tartar, celluloid, oxalates, tartaric acid, purified methyl alcohol, glycerol, and stable hydrogen cyanide. It is also used in the photographic, ceramic, metallurgic, rubber, leather, engraving, pharmaceutical, paper, and lithographic industries.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. If victim is conscious, administer water ormilk. Do not induce vomiting. Medical observation isrecommended for 24-48 h after breathing overexposure, aspulmonary edema may be delayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consideradministering a corticosteroid spray.
Source
Oxalic acid occurs naturally in many plants including buckwheat leaves (111,000 ppm),
lambsquarter (140,000 to 300,000 ppm), black pepper (4,000 to 34,000 ppm), star fruit (50,000 to
95,800 ppm), purslane (1,679 to 16,790 ppm), nance bark (27,300 ppm), rhubarb 4,400 to 13,360
ppm), tea leaves (2,192 to 10,000 ppm), bitter lettuce (10,000 ppm), spinach (6,580 ppm), cacao
(1,520 to 5,000 ppm), bananas (22 to 5,240 ppm), ginger (5,000 ppm in rhizome), cashews (3,184
ppm), almonds (4,073 ppm), taro roots (1,334 ppm), tamarind (1,960 ppm), garden sorrel (3,000
ppm), mustard green leaves (1,287 ppm), peppers (257 to 1,171 ppm), sweet potato roots (1,000
ppm), pumpkins, oats (400 ppm), tomatillo (109 to 536 ppm), various cabbage leaves (59 to 350
ppm), and horseradish (Duke, 1992).
Oxalic acid was identified as a constituent in a variety of composted organic wastes. Detectable
concentrations were reported in all 21 composts extracted with water. Concentrations ranged from
0.60 mmol/kg in a straw + dairy cattle manure to 21.89 mmol/kg in straw + wood bark + dairy
cattle manure. The overall average concentration was 9.67 mmol/kg (Baziramakenga and Simard,
1998).
Environmental Fate
Biological. Heukelekian and Rand (1955) reported a 5-d BOD value of 0.12 g/g which is 66.7%
of the ThOD value of 0.18 g/g.
Chemical/Physical. At temperatures greater than 189.5 °C, decomposes to carbon dioxide,
carbon monoxide, formic acid, and water (Windholz et al., 1983). Ozonolysis of oxalic acid in
distilled water at 25 °C under acidic conditions (pH 6.3) yielded carbon dioxide (Kuo et al., 1977).
Absorbs moisture in air forming the dihydrate (Huntress and Mulliken, 1941).
Reacts with bases forming water soluble salts.
storage
Color Code—White: Corrosive or Contact Hazard;Store separately in a corrosion-resistant location. Prior toworking with this chemical you should be trained on itsproper handling and storage. Oxalic acid must be stored toavoid contact with silver or strong oxidizers (such as chlorine and bromine) because violent reactions occur. Store intightly closed containers in a cool, well-ventilated areaaway from heat. Sources of ignition, such as smoking andopen flames, are prohibited where oxalic acid is used, handled, or stored in a manner that could create a potential fireor explosion hazard.
Shipping
UN3261 Corrosive solid, acidic, organic, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name Required.
Incompatibilities
The aqueous solution is a medium-strong acid. Compounds of the carboxyl group react with all bases, both inorganic and organic (i.e., amines) releasing substantial heat, water and a salt that may be harmful. Incompatible with arsenic compounds (releases hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides (releasing heat, toxic, and possibly flammable gases), thiosulfates and dithionites (releasing hydrogen sulfate and oxides of sulfur). Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from silver compounds; strong alkalis; chlorites. Contact with some silver compounds forms explosive materials.
Waste Disposal
Pretreatment involves chemical reaction with limestone or calcium oxide forming calcium oxalate. This may then be incinerated utilizing particulate collection equipment to collect calcium oxide for recycling.
Oxalic acid Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
1of6
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20293 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 57511 | 58 |
| Hebei Fengmu Trading Co., Ltd. | +8613393234347 | Lyla@fengmuchem.com | China | 2980 | 58 |
| Hebei Andu Technology Com.,Ltd | +86-86-17798073498 +8617798073498 | admin@hbandu.com | China | 300 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12446 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5873 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5882 | 58 |
| Hebei Fengjia New Material Co., Ltd | +86-0311-87836622 +86-17333973358 | sales06@hbduling.cn | China | 8056 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 | lisa@kingfinertech.com | China | 2990 | 58 |
Related articles
- Oxalic Acid: A Comprehensive Overview
- Oxalic acid, is an organic compound that is widely used in various industrial and chemical processes.
- May 31,2024
- Health Hazard of Oxalic acid
- Oxalic acid is an organic acid with the IUPAC name ethanedioic acid and formula HO2C?CO2H. It is the simplest dicarboxylic aci....
- Apr 12,2022
View Lastest Price from Oxalic acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-09-21 | Oxalic Acid Dihydrate
144-62-7
|
US $3.60 / kg | 1kg | ≥99% | 3000tons/month | Hebei Andu Technology Com.,Ltd | |
 |
2024-09-20 | Oxalic acid
144-62-7
|
US $99.00-66.00 / kg | 0.0010000000474974513kg | 99% | 5000 | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | |
 |
2024-09-19 | Oxalic acid
144-62-7
|
US $1.00 / kg | 10kg | 99 % | 10000kg | Hebei Chuanghai Biotechnology Co,.LTD |
-

- Oxalic Acid Dihydrate
144-62-7
- US $3.60 / kg
- ≥99%
- Hebei Andu Technology Com.,Ltd
-

- Oxalic acid
144-62-7
- US $99.00-66.00 / kg
- 99%
- HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
-

- Oxalic acid
144-62-7
- US $1.00 / kg
- 99 %
- Hebei Chuanghai Biotechnology Co,.LTD