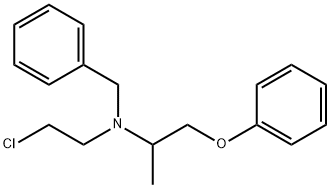
phenoxybenzamine
- CAS No.
- 59-96-1
- Chemical Name:
- phenoxybenzamine
- Synonyms
- Phenoxy;Dibenyline;Phenoxybenzamin;phenoxybenzamine USP/EP/BP;N-Benzyl-N-(2-chloroethyl)-1-phenoxypropan-2-aMine;N-(2-Chloroethyl)-N-(1-methyl-2-phenoxyethyl)benzylamine;BENZYLAMINE,N-(2-CHLOROETHYL)-N-(1-METHYL-2-PHENOXYETHYL)-;Benzenemethanamine, N-(2-chloroethyl)-N-(1-methyl-2-phenoxyethyl)-
- CBNumber:
- CB1933947
- Molecular Formula:
- C18H22ClNO
- Molecular Weight:
- 303.83
- MDL Number:
- MFCD00599580
- MOL File:
- 59-96-1.mol
| Melting point | 38-40° |
|---|---|
| Boiling point | 381.5±27.0 °C(Predicted) |
| Density | 1.0513 (rough estimate) |
| refractive index | 1.5600 (estimate) |
| pka | 6.58±0.50(Predicted) |
| color | Crystals from pet ether |
| CAS DataBase Reference | 59-96-1 |
| FDA UNII | 0TTZ664R7Z |
| EPA Substance Registry System | N-(2-Chloroethyl)-N-(1-methyl-2-phenoxyethyl)benzenemethanamine (59-96-1) |
phenoxybenzamine price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| American Custom Chemicals Corporation | API0008677 | PHENOXYBENZAMINE 95.00% | 59-96-1 | 100MG | $885.31 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0008677 | PHENOXYBENZAMINE 95.00% | 59-96-1 | 1G | $1124.55 | 2021-12-16 | Buy |
| Crysdot | CD12057082 | N-Benzyl-N-(2-chloroethyl)-1-phenoxypropan-2-amine 95+% | 59-96-1 | 1g | $673 | 2021-12-16 | Buy |
phenoxybenzamine Chemical Properties,Uses,Production
Originator
Dibenzyline, SKF, US ,1953
Uses
Antihypertensive.
Definition
ChEBI: Phenoxybenzamine is an aromatic amine.
Manufacturing Process
Step 1: In a 500 ml flask equipped with gas inlet tube, dropping funnel and reflux condenser is placed 139 grams of 1-phenoxy-2-propanol. A stream of dry air is bubbled through the alcohol while 55 grams of thionyl chloride is added dropwise with external cooling. The stream of dry air is continued for about six hours or until most of the hydrogen chloride has been expelled and then another 55 grams of thionyl chloride is added. The reaction mixture is allowed to stand twenty-four hours, a few drops of pyridine are added and the mixture heated 4 hours on the steam bath. The cooled reaction mixture is poured into water, the crude product is washed with dilute sodium bicarbonate solution and finally taken up in benzene. The benzene is distilled at ordinary pressure and the residue distilled in vacuo to yield 60-70% of 1-phenoxy-2chloropropane, BP 93°-94°C/5 mm.
Step 2: To 494 grams of ethanolamine, heated to approximately 150°C in a 500 ml flask equipped with stirrer, condenser and dropping funnel, is added 465 grams of 1-phenoxy-2-chloropropane with mechanical stirring. The reaction mixture is then heated to reflux for 3 hours, cooled and poured into a liter of water. The organic layer is extracted into ether and the ether solution is extracted with dilute hydrochloric acid. The aqueous acid solution is then made alkaline with 40% sodium hydroxide solution and the organic base is extracted into ether. Removal of the ether leaves N-(phenoxyisopropyl)ethanolamine which, after recrystallization from hexane, melts at 70.5°-72°C.
Step 3: To 43 grams of N-(phenoxyisopropyl)ethanolamine dissolved in 500 ml of alcohol in a 1,000 ml flask equipped with stirrer and condenser is added 28 grams of benzyl chloride and 18.5 grams of sodium bicarbonate. The mixture is stirred and refluxed for 10 hours and then approximately half the alcohol is removed by distillation. The remaining solution is poured into 500 ml of water and the organic material extracted with 3 100-ml portions of ether. The combined ether extracts are washed with water, dried over anhydrous potassium carbonate and filtered. After removal of the ether, the residue is distilled in vacuo to yield N-(phenoxyisopropyl)-Nbenzylethanolamine, BP 163°-168°C/0.2 mm.
Step 4: A solution of 20 grams of the above amino alcohol is dissolved in 50 ml of dry chloroform and treated with dry hydrogen chloride until acid. Then a solution of 9 grams of thionyl chloride in 50 ml of dry chloroform is added and the reaction mixture is heated on a water bath at 50°-60°C for 2 hours. Most of the chloroform is removed by distillation under reduced pressure. Addition of ether to the residue causes the product to crystallize. After recrystallization from a mixture of alcohol and ether, the N-(phenoxyisopropyl)-N-benzyl-βchloroethylamine hydrochloride melts at 137.5°-140°C.
brand name
Dibenzyline (WellSpring);Dibenzyran.
Therapeutic Function
Adrenergic blocker
World Health Organization (WHO)
Phenoxybenzamine, a long-acting alpha-adrenoreceptor antagonist, was introduced in 1953 and has been used in a variety of peripheral vascular disorders. In 1982 it was shown to have mutagenic activity and in 1985 it was found to be carcinogenic in the rat. Its approved use was subsequently restricted by several regulatory authorities and phenoxybenzamine is currently used to manage hypertensive episodes associated with phaeochromocytoma, as an adjunct to the short-term management of urinary retention due to neurogenic bladder, in the short-term treatment of benign prostatic hypertrophy in patients awaiting surgery, and in inoperable benign prostatic hypertrophy.
Safety Profile
Confirmed carcinogen with experimental carcinogenic and neoplastigenic data. Poison by intravenous and intracerebral routes. Moderately toxic by ingestion. Human reproductive effects by ingestion: spermatogenesis. Experimental reproductive effects. When heated to decomposition it emits very toxic fumes of Cland NOx.
Purification Methods
The free base is crystallised from pet ether, and the HCl is crystallised from EtOH/diethyl ether. [Beilstein 12 IV 2204.]
phenoxybenzamine Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shaanxi Dideu Medichem Co. Ltd | +86-029-81138252 +86-18789408387 | 1057@dideu.com | China | 3537 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9320 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29220 | 58 |
| Finetech Industry Limited | +86-27-87465837 +8618971612321 | info@finetechnology-ind.com | China | 9702 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | CHINA | 52867 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 49999 | 58 |
| Beijing HuaMeiHuLiBiological Chemical | 010-56205725 | waley188@sohu.com | China | 12338 | 58 |
| TargetMol Chemicals Inc. | 021-33632979 15002134094 | marketing@targetmol.com | China | 7934 | 58 |
| Finetech Industry Limited | 027-87465837 19945049750 | sales@finetechnology-ind.com | China | 9664 | 58 |
View Lastest Price from phenoxybenzamine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-08-13 | phenoxybenzamine USP/EP/BP
59-96-1
|
US $1.10 / g | 1g | 99.9% | 100 Tons min | Dideu Industries Group Limited | |
 |
2020-05-12 | PhenoxybenzaMine Hydrochloride
59-96-1
|
US $1.00 / KG | 1KG | 99% | 20T | Shaanxi Dideu Medichem Co. Ltd |
-

- phenoxybenzamine USP/EP/BP
59-96-1
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited
-

- PhenoxybenzaMine Hydrochloride
59-96-1
- US $1.00 / KG
- 99%
- Shaanxi Dideu Medichem Co. Ltd