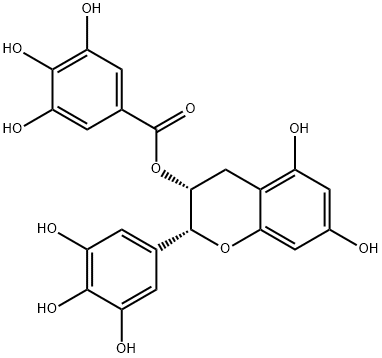
(-)-Epigallocatechin gallate
- CAS No.
- 989-51-5
- Chemical Name:
- (-)-Epigallocatechin gallate
- Synonyms
- EGCG;EPIGALLOCATECHIN GALLATE;ECGC;(-)-Epigallocatechin-3-O-gallate (20 mg);epigallocatechin3-gallate;Epigallocatechin-3-Monogallate;(-)-epigallocatechin 3-gallate;E-5187;(EGCE);EGCG-d6
- CBNumber:
- CB2227188
- Molecular Formula:
- C22H18O11
- Molecular Weight:
- 458.37
- MDL Number:
- MFCD00075940
- MOL File:
- 989-51-5.mol
- MSDS File:
- SDS
| Melting point | 222-224°C |
|---|---|
| alpha | D -185 ±2°(ethanol) |
| refractive index | -175.5 ° (C=1, EtOH) |
| storage temp. | 2-8°C |
| solubility | H2O: ≥5mg/mL, clear |
| Boiling point | 909.1±65.0 °C(Predicted) |
| Density | 1.90±0.1 g/cm3(Predicted) |
| pka | 7.75±0.25(Predicted) |
| color | White to Light Brown |
| Water Solubility | Soluble in ethanol, dimethyl formamide, water. |
| Merck | 14,3526 |
| Stability | Stable, but may be light sensitive. Incompatible with strong oxidizing agents. |
| InChIKey | WMBWREPUVVBILR-WIYYLYMNSA-N |
| LogP | 0.639 (est) |
| CAS DataBase Reference | 989-51-5(CAS DataBase Reference) |
| EWG's Food Scores | 2 |
| NCI Dictionary of Cancer Terms | EGCG; epigallocatechin-3-gallate |
| FDA UNII | BQM438CTEL |
| NCI Drug Dictionary | epigallocatechin gallate |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H317-H319-H411 | |||||||||
| Precautionary statements | P261-P273-P280-P301+P312-P302+P352-P305+P351+P338 | |||||||||
| Safety Statements | 24/25 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | KB5200000 | |||||||||
| F | 10-23 | |||||||||
| HS Code | 29339900 | |||||||||
| Toxicity | LD50 oral in mouse: 2170mg/kg | |||||||||
| NFPA 704 |
|
(-)-Epigallocatechin gallate price More Price(60)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | PHL89656 | (−)-Epigallocatechin3-gallate phyproof?ReferenceSubstance | 989-51-5 | 50mg | $382 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1236700 | (?)-Epigallocatechin gallate United States Pharmacopeia (USP) Reference Standard | 989-51-5 | 20mg | $394 | 2024-03-01 | Buy |
| Sigma-Aldrich | 03970590 | Epigallocatechin gallate primary pharmaceutical reference standard | 989-51-5 | 10mg | $364 | 2024-03-01 | Buy |
| TCI Chemical | E0694 | (-)-Epigallocatechin Gallate Hydrate >98.0%(HPLC) | 989-51-5 | 100mg | $99 | 2024-03-01 | Buy |
| TCI Chemical | E0694 | (-)-Epigallocatechin Gallate Hydrate >98.0%(HPLC) | 989-51-5 | 500mg | $273 | 2024-03-01 | Buy |
(-)-Epigallocatechin gallate Chemical Properties,Uses,Production
Uses
(-)-Epigallocatechin Gallate is a tumor-inhibiting constituent of green tea. (-)-Epigallocatechin Gallate alters the cleavage of amyloid precursor protein, decreasing production of amaloid-ß and amaloid plaques in mice. This compound has neuroprotective properties.
Chemical Properties
solid
Uses
(-)-Epigallocatechin Gallate is a tumor-inhibiting constituent of green tea. (-)-Epigallocatechin Gallate alters the cleavage of amyloid precursor protein, decreasing production of amaloid-β and amaloid plaques in mice. This compound has neuroprotective properties.
Uses
A tumor-inhibiting constituent of green tea. Alters the cleavage of amyloid precursor protein, decreasing production of amaloid- and amaloid plaques in mice
Uses
An inhibitor of Bcl-2 and NOS2
Uses
telomerase inhibitor
Definition
ChEBI: (-)-epigallocatechin 3-gallate is a gallate ester obtained by the formal condensation of gallic acid with the (3R)-hydroxy group of (-)-epigallocatechin. It has a role as an antineoplastic agent, an antioxidant, a Hsp90 inhibitor, a neuroprotective agent, a plant metabolite, a geroprotector and an apoptosis inducer. It is a gallate ester, a polyphenol and a member of flavans. It is functionally related to a (-)-epigallocatechin.
General Description
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Epigallocatechin gallate is a potent polyphenolic flavonoid which is found as a component of tea. It exhibits antioxidant, antimutagenic, antitumor and anti-inflammatory properties, thereby contributing to the health-beneficial actions. It finds potential use as a drug candidate in the pharmaceutical, cosmetic, and nutritional fields.
Hazard
Moderately toxic by ingestion.
Biological Activity
(-)-epigallocatechin gallate (egcg), the major catechin accounting for 59% of the total catechins in green tea, is a powerful antioxidant as well as an antiangiogenic and antitumor agent. egcg has been studied for its role in the chemoprevention of a wild range of cancers, including liver, stomach, skin, lung, mammary gland and colon cancers. study results show that egcg is able to induce apoptosis, promote cell growth arrest and block carcinogenesis by affecting signal transduction pathways. moreover, egcg exhibits inhibition against a variety of viruses, including hcv, hiv-1, hbv, hsv-1, hsv-2, ebv, adenovirus, influenza virus and enterovirus, as well as several enzymes, including dnmts, proteases and dhfr.singh bn, shankar s, srivastava rk. green tea catechin, epigallocatechin-3-gallate (egcg): mechanisms, perspectives and clinical applications. biochem pharmacol. 2011; 82(12):1807-1821.steinmann j, buer j, pietschmann t, steinmann e. anti-infective properties of epigallocatechin-3-gallate (egcg), a component of green tea. br j pharmacol. 2013; 168(5):1059-1073
Biochem/physiol Actions
(-)-Epigallocatechin gallate (EGCG), an antioxidant polyphenol flavonoid exerts anti-tumor properties by inhibiting telomerase and DNA methyltransferase activity. EGCG inhibits the expression of matrix metalloproteinase-2 (MMP-2), MMP-9 and reduces the invasiveness. EGCG blocks the activation of epidermal growth factor (EGF) receptors and human epidermal growth factor receptor-2 (HER-2). EGCG increases bone mineral density and reduces bone resorption. EGCG inhibits osteoclastogenesis by inhibiting receptor activator of nuclear factor κ-B ligand (RANKL) induced nuclear factor κ B (NF-κB) transcriptional activity. EGCG reduces skeletal muscle atrophy. EGCG has anti-aging property and increases myogenic differentiation. EGCG inhibits fatty acid synthase and glutamate dehydrogenase activity.
Anticancer Research
EGCG is an ester of gallic acid and epigallocatechin and is a catechin compound(Murakami et al. 1996). It is found most abundantly in green tea. It can be used to treat brain, prostate, cervical, and bladder cancers (Wang et al. 2012). It suppressesthe ornithine decarboxylase action, an enzyme that leads to rapid proliferation andfurthermore circumvents apoptosis (Singh et al. 2016a). It suppresses nuclear factor(NF-κB) activation and expression of Bcl-2 (B-cell lymphoma 2) as well as COX-2(cyclooxygenase-2) in prostate cancer cells and causes induction of apoptosis. Ithamper the matrix metallopeptidase-9 (MMP-9) activation in bladder and lungcancer cells and suppresses the synthesis of VGEF (vascular endothelial growthfactor) in head and neck cancers. It prevents ERK (extracellular signal-regulatedkinase) phosphorylation and MMP-2 and MMP-9 activation and suppresses ERK,c-Jun N-terminal kinase (JNK), and MMP-9 expressions in gastric carcinoma cells(Singh et al. 2016a). It is binding and inhibits the antiapoptotic protein Bcl-xL,interferes with EGFR (epidermal growth factor receptor) signaling, and inhibitshepatocyte growth factor-induced cell proliferation and MAPK (mitogen-activatedprotein kinase), CDK (cyclin-dependent kinase), and cell signaling linked to growthfactors (Wang et al. 2012; Du et al. 2012).
Green tea constitutes the rich amount of EGCG which aids in cancer chemoprevention(Fujiki et al. 1998). EGCG improved the impacts of ginseng compoundin the restraint of colon tumor cell development, showing that green tea could bea successful synergist with an anticancer agent for malignancy chemoprevention.It obstructs the PDGF-initiated proliferation and migration of rodent pancreaticstellate cells (Masamune et al. 2005). The soluble and plasma membrane-integratedEGCG straightforwardly communicates with PDGF-BB and in this wayputs off precise receptor binding promoting the inhibitory impacts of EGCG onplatelet-derivedgrowth factor-incited cell signaling and mitogens (Weber et al.2004).
Anticancer Research
EGCG and EGC are the active polyphenol compounds found in green tea, found toinhibit p-glycoprotein transport activities in Chinese hamster ovary (p-gp+) cells.EGCG facilitates the retraction of MDR phenotype by reducing cellular drug effluxwhen given in combination with vinblastine or doxorubicin. Hesperetin, quercetin,daidzein, silymarin, naringenin, and resveratrol also inhibit the MRP1, MRP4, andMRP5 (Kawasaki et al. 2008). Curcumin increases the cellular accumulation ofanticancer agents like cisplatin, tamoxifen, daunorubicin, vincristine, anddoxorubicin and thereby effectively sensitizes the drug-resistant cancer cells. Areduction in MDR1B expression in L1210/Adr cells (mouse leukemic MDR cells)by curcumin is mediated by PI3K, Akt, and NF-κB pathways. It also inhibits theABCG2 transporter activity. In addition curcumin facilitates the accumulation ofmitoxantrone and doxorubicin in ABCG2-expressing HEK cells and hence reversesMDR (Kawasaki et al. 2008; Dandawate et al. 2013).
storage
Store at -20°C
(-)-Epigallocatechin gallate Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
| Chengdu ChenLv Herb Co.,Ltd | +undefined13608205856 | maryextract@126.com | China | 127 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Wuhan Haorong Biotechnology Co.,ltd | +8618565342920 | sales@chembj.net | China | 269 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 | qinhe02@xaltbio.com | China | 1000 | 58 |
| Chongqing Zhihe Biopharmaceutical Co., Ltd. | +86-18580541567 +86-17782035140 | sales@zhswyy.com | China | 338 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 476 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
Related articles
- Source and effects of EGCG
- Phytochemicals are biologically active plant-derived compounds and are thought to be responsible for the health-promoting effe....
- Mar 3,2022
View Lastest Price from (-)-Epigallocatechin gallate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-26 | (-)-Epigallocatechin gallate
989-51-5
|
US $0.00-0.00 / kg | 0.10000000149011612kg | ≥98% | 20tons | Chongqing Zhihe Biopharmaceutical Co., Ltd. | |
 |
2024-04-26 | (-)-Epigallocatechin gallate
989-51-5
|
US $1.00 / KG | 1KG | 99.91% | 200000 | Ouhuang Engineering Materials (Hubei) Co., Ltd | |
 |
2024-04-24 | (-)-Epigallocatechin gallate
989-51-5
|
US $0.00-0.00 / kg | 20kg | 99% | 200000 | Wuhan Haorong Biotechnology Co.,Ltd |
-

- (-)-Epigallocatechin gallate
989-51-5
- US $0.00-0.00 / kg
- ≥98%
- Chongqing Zhihe Biopharmaceutical Co., Ltd.
-

- (-)-Epigallocatechin gallate
989-51-5
- US $1.00 / KG
- 99.91%
- Ouhuang Engineering Materials (Hubei) Co., Ltd
-

- (-)-Epigallocatechin gallate
989-51-5
- US $0.00-0.00 / kg
- 99%
- Wuhan Haorong Biotechnology Co.,Ltd