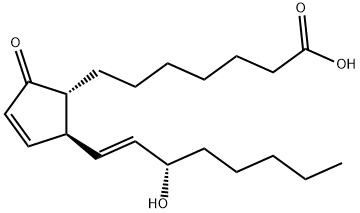
PROSTAGLANDIN A1
- CAS No.
- 14152-28-4
- Chemical Name:
- PROSTAGLANDIN A1
- Synonyms
- PGA1;Prostaglandin A;Prostagladin E1;C04685;pga(sub1);pga(sup1);Prostagladin A;PROSTAGLANDIN A1;PROSTAGLANDINS A1;prostaglandina(sup1)
- CBNumber:
- CB3666883
- Molecular Formula:
- C20H32O4
- Molecular Weight:
- 336.47
- MDL Number:
- MFCD00077858
- MOL File:
- 14152-28-4.mol
- MSDS File:
- SDS
| Melting point | 43°C |
|---|---|
| Boiling point | 392.93°C (rough estimate) |
| Density | 1.0192 (rough estimate) |
| refractive index | 1.4434 (estimate) |
| storage temp. | -20°C |
| solubility | acetone: 10 mg/mL, clear |
| form | White solid. |
| pka | 4.77±0.10(Predicted) |
| color | Off-white |
| BRN | 2003401 |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 1 month. |
| FDA UNII | VYR271N44P |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H360F | |||||||||
| Precautionary statements | P201-P301+P312+P330-P308+P313 | |||||||||
| Safety Statements | 22-24/25 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | GY5977900 | |||||||||
| F | 8-10 | |||||||||
| HS Code | 2937500000 | |||||||||
| NFPA 704 |
|
PROSTAGLANDIN A1 price More Price(17)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | P7265 | Prostaglandin A1 synthetic | 14152-28-4 | 1mg | $161 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1578500 | Prostaglandin A1 United States Pharmacopeia (USP) Reference Standard | 14152-28-4 | 25mg | $3090 | 2024-03-01 | Buy |
| Cayman Chemical | 10010 | Prostaglandin A1 ≥98% | 14152-28-4 | 1mg | $36 | 2024-03-01 | Buy |
| Cayman Chemical | 10010 | Prostaglandin A1 ≥98% | 14152-28-4 | 5mg | $120 | 2024-03-01 | Buy |
| Sigma-Aldrich | P7265 | Prostaglandin A1 synthetic | 14152-28-4 | 5mg | $559 | 2024-03-01 | Buy |
PROSTAGLANDIN A1 Chemical Properties,Uses,Production
Description
Prostaglandin A1 (PGA1) was first isolated as a dehydration product of the PGE1 compounds found in human semen. It has been shown to cause renal vasodilation, increased urine sodium excretion, and lowered arterial pressure in hypertensive patients. It also has growth-
Uses
Prostaglandin A1 has been shown to inhibit human immunodeficiency virus type 1 (HIV-1) replication in various cell types. In addition, as a precursor to the anti-inflammatory prostaglandins, it activates pro-inflammatory PGD2 receptor CRTH2 initiating the anti-inflammatory response.
Definition
ChEBI: Prostaglandin A1 is a prostaglandins A. It is a conjugate acid of a prostaglandin A1(1-).
General Description
Prostaglandin A1(PGA1) belongs to the class of cyclopentenone prostaglandin. PGA1 is generated by the dehydration of prostaglandin E1 (PGE1).
Biochem/physiol Actions
Prostaglandin A1 (PGA1) boosts the expression of human liver-specific cytochrome P450 4F3B (CYP4F3B) and stimulates the synthesis of 20-hydroxy-eicosatetraenoic acids (HETEs). It has an ability to stimulate cell differentiation, ferritin synthesis and heat shock response in human cells. PGA1 exhibits antitumor and antiviral activities. In addition, it also elicits anti-inflammatory effects by activating peroxisome proliferator-activated receptor (PPAR). Elevated levels of PGA1 has been observed in hypertension patients.
Enzyme inhibitor
This eicosanoid (FWfree-acid = 336.47 g/mol; CAS 14152-28-4; Symbol: PGA1) induces apoptosis and stimulates the expression of stress genes. The structure and role of the prostaglandin A1 was investigated by Bergstr?m and Samuelsson, who shared the 1982 Nobel Prize in Physiology or Medicine for their pioneering work on the eicosanoids. Target(s): CYP4F2; DNA topoisomerase II; HIV-1 transcription; 3ahydroxysteroid dehydrogenase, NADP+-dependent; IkB kinase; Mayaro virus replication; stress-induced NF-kB activation; and virus protein biosynthesis, or translation.
References
1) Lee et al. (1972) Renal effects of prostaglandin A1 in patients with essential hypertension; Kidney Int. 1 254 2) Toppozada et al. (1988) Treatment of preeclampsia with prostaglandin A1; Am. J. Obstet. Gynecol. 159 160 3) Rossi et al. (2000) Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase; Nature 403 103 4) Mandal et al. (2005), Yin-yang: balancing act of prostaglandins with opposing functions to regulate inflammation; J. Immunol. 175 6271 5) Carta et al. (2014) Prostaglandin A1 inhibits avian influenza virus replication at a postentry level: Effect on virus protein synthesis and NF-kB activity; Prostaglandins Leukot. Essential Fatty Acids 91 311 6) Tsukimoto et al. (2015) A new role for PGA1 in inhibiting hepatitis C virus-IRES-mediated translation by targeting viral translation factors; Antiviral Res. 117 1 7) Diez-Dacal et al. (2011) Identification of aldo-keto reductase AKR1B10 as a selective target for modification and inhibition by prostaglandin A(1): implications for antitumoral activity; Cancer Res. 71 4161 8) Anta et al. (2016), PGA1-induced apoptosis involves specific activation of H-Ras and N-Ras in cellular endomembranes; Cell Death Dis. 7 e2311
PROSTAGLANDIN A1 Preparation Products And Raw materials
Raw materials
Preparation Products
PROSTAGLANDIN A1 Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 992 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 43348 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 57511 | 58 |
| Sinopharm Chemical Reagent Co,Ltd. | 86-21-63210123 | sj_scrc@sinopharm.com | China | 9823 | 79 |
| BOC Sciences | 1-631-485-4226; 16314854226 | info@bocsci.com | United States | 14059 | 65 |
| Shanghai Kewel Chemical Co., Ltd. | 021-64609169 18901607656 | greensnown@163.com | China | 9911 | 50 |
| Sigma-Aldrich | 021-61415566 800-8193336 | orderCN@merckgroup.com | China | 51471 | 80 |
| BOC Sciences | 16314854226 | info@bocsci.com | United States | 9926 | 65 |
| Shanghai EFE Biological Technology Co., Ltd. | 021-65675885 18964387627 | info@efebio.com | China | 9709 | 58 |
View Lastest Price from PROSTAGLANDIN A1 manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-22 | Prostagladin E1
14152-28-4
|
US $0.00 / Kg/Bag | 2Kg/Bag | 99% up, High Density | 20 tons | Sinoway Industrial co., ltd. | |
 |
2020-02-20 | PROSTAGLANDIN A1
14152-28-4
|
US $1.00 / KG | 1KG | 99% | 200kgs | Career Henan Chemical Co |
-

- Prostagladin E1
14152-28-4
- US $0.00 / Kg/Bag
- 99% up, High Density
- Sinoway Industrial co., ltd.
-

- PROSTAGLANDIN A1
14152-28-4
- US $1.00 / KG
- 99%
- Career Henan Chemical Co