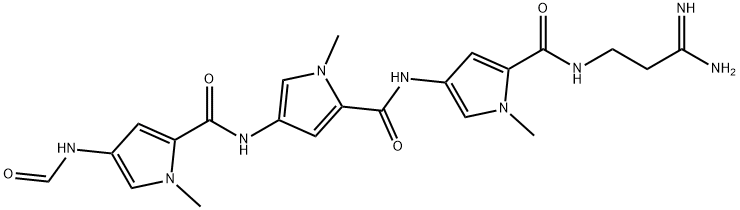
Stallimycin
- CAS No.
- 636-47-5
- Chemical Name:
- Stallimycin
- Synonyms
- Distamycin;Stallimycin;Distamycin-3;3-[4-[4-[4-(Formylamino)-1-methyl-1H-pyrrole-2-ylcarbonylamino]-1-methyl-1H-pyrrole-2-ylcarbonylamino]-1-methyl-1H-pyrrole-2-ylcarbonylamino]propanamidine;N-[5-[[(3-Amino-3-iminopropyl)amino]carbonyl]-1-methyl-1H-pyrrol-3-yl]-4-[[[4-(formylamino)-1-methyl-1H-pyrrol-2-yl]carbonyl]amino]-1-methyl-1H-pyrrole-2-carboxamide;1H-Pyrrole-2-carboxamide, N-[5-[[(3-amino-3-iminopropyl)amino]carbonyl]-1-methyl-1H-pyrrol-3-yl]-4-[[[4-(formylamino)-1-methyl-1H-pyrrol-2-yl]carbonyl]amino]-1-methyl-
- CBNumber:
- CB51179775
- Molecular Formula:
- C22H27N9O4
- Molecular Weight:
- 481.51
- MDL Number:
- MOL File:
- 636-47-5.mol
| Melting point | 154-156° |
|---|---|
| Boiling point | 579.2°C (rough estimate) |
| Density | 1.3298 (rough estimate) |
| refractive index | 1.6910 (estimate) |
| EWG's Food Scores | 1 |
| FDA UNII | 80O63P88IS |
Stallimycin Chemical Properties,Uses,Production
Originator
Herperal,Farmitalia,Italy,1978
Manufacturing Process
A spore suspension obtained upon washing a culture of Streptomyces
distallicus is added to 3,000 ml of a sterile medium consisting of the
following:Dextrose 2%
Corn steep liquor extract 2%
CaCO3 1%
(NH4)2SO4 0.3%
NaCl 0.3%Fermentation is continued at 28°C for 40 hours at a stirring rate of 150 to 250
rpm and a rate of air flow of 1 to 2 l/min/l of culture medium.
300 ml of a suspension of the vegetative mycelium of this culture are used for
inoculating 6,000 ml of a similar sterile culture medium. At this production
stage, the culture is kept fermenting for 85 to 100 hours (pH 7.6 at 28°C) at
a stirring rate of 350 to 450 rpm and a rate of air flow of 1 to 1.5 l/min/l of
culture medium.
To 17 l of a culture obtained by submerged fermentation as mentioned above,
siliceous earth is added and the batch is filtered. The mixture of mycelium and
the siliceous earth are agitated for 1 hour with 2.5 l of butanol. This treatment
is repeated twice. The butanolic extracts are combined, washed with water,
evaporated to dryness (about 10 g) and boiled with acetone (80 ml). The 5 g of distamycin is extracted six times with ethanol. The ethanolic extracts
are combined, concentrated and filtered through a column containing 70 g of
alumina. Elution is carried out with the same solvent. The effluent (central
fractions) is collected and evaporated to dryness to yield 0.43 g of pure
distamycin A: decomposition point, 183°C to 185°C. The product can be
further purified by crystallization from aqueous n-butanol.
Therapeutic Function
Antibiotic
Stallimycin Preparation Products And Raw materials
Stallimycin Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| BOC Sciences | 1-631-485-4226; 16314854226 | info@bocsci.com | United States | 14059 | 65 |
| ChemStrong Scientific Co.,Ltd | 0755-0755-66853366 13670046396 | sales@chem-strong.com | China | 18021 | 56 |
| Zhejiang Huida Biotech Co., LTD | 0571-89903882 13626641628 | jiangnan@huidabiotech.com | China | 3656 | 58 |
| Zhejiang Huida Biotech Co., LTD | 0571-0571-89903882 15990081639 | sunshixuan@huidabiotech.com | China | 3705 | 58 |
| Hangzhou Hesheng Taiyi Biotechnology Co. , Ltd. | 15868607770 | hstysales@126.com | China | 1165 | 58 |
| Shanghai Yanchun Biopharmaceutical Technology Co., Ltd | 4001-009266 17349750668 | product@sci-e.com | China | 2811 | 58 |