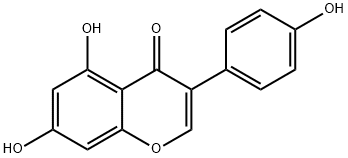
Genistein
- CAS No.
- 446-72-0
- Chemical Name:
- Genistein
- Synonyms
- Licoisoflavone;5,7-Dihydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one;GEN;GENESTEIN;Bonistein;prunetol;GeniVida;G2535;BIO-00;BIO300
- CBNumber:
- CB6163787
- Molecular Formula:
- C15H10O5
- Molecular Weight:
- 270.24
- MDL Number:
- MFCD00016952
- MOL File:
- 446-72-0.mol
- MSDS File:
- SDS
| Melting point | 297-298 °C |
|---|---|
| Boiling point | 333.35°C (rough estimate) |
| Density | 1.2319 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| storage temp. | -20°C |
| solubility | DMSO: soluble |
| form | powder |
| pka | 6.51±0.20(Predicted) |
| color | off-white |
| Water Solubility | insoluble |
| Merck | 14,4391 |
| BRN | 263823 |
| Stability | Light Sensitive |
| InChIKey | TZBJGXHYKVUXJN-UHFFFAOYSA-N |
| LogP | 3.114 (est) |
| CAS DataBase Reference | 446-72-0(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| NCI Dictionary of Cancer Terms | genistein |
| FDA UNII | DH2M523P0H |
| NCI Drug Dictionary | genistein |
| EPA Substance Registry System | 4H-1-Benzopyran-4-one, 5,7-dihydroxy-3-(4-hydroxyphenyl)- (446-72-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P264-P270-P301+P312-P501 | |||||||||
| Hazard Codes | Xi,Xn | |||||||||
| Risk Statements | 36/38-22 | |||||||||
| Safety Statements | 26-24/25-22 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | NR2392000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | IRRITANT | |||||||||
| HS Code | 29329990 | |||||||||
| NFPA 704 |
|
Genistein price More Price(70)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 345834 | Genistein, Soybean - CAS 446-72-0 - Calbiochem A cell-permeable, reversible, substrate competitive inhibitor of protein tyrosine kinases, including autophosphorylation of epidermal growth factor receptor kinase (IC?? = 2.6 μM). | 446-72-0 | 50mg | $272 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1288816 | Genistein United States Pharmacopeia (USP) Reference Standard | 446-72-0 | 15mg | $354 | 2024-03-01 | Buy |
| Sigma-Aldrich | 05360590 | Genistein primary reference standard | 446-72-0 | 50mg | $464 | 2024-03-01 | Buy |
| TCI Chemical | G0272 | Genistein >98.0%(HPLC) | 446-72-0 | 100mg | $82 | 2024-03-01 | Buy |
| TCI Chemical | G0272 | Genistein >98.0%(HPLC) | 446-72-0 | 1g | $290 | 2024-03-01 | Buy |
Genistein Chemical Properties,Uses,Production
description
Genistein is one of the most abundant isoflavones in soy and is one of several known isoflavones. Isoflavones compounds, such as genistein and daidzein, are found in a number of plants, but soybeans and soy products like tofu and textured vegetable protein are the primary food source. Because of its similar structure to that of human estrogen it is also called a phytoestrogen. Genistein is a natural bioactive compound derived from legumes and has drawn because of its potentially beneficial effects on some human degenerative diseases. It has a weak estrogenic effect and is a well-known non-specific tyrosine kinase inhibitor at pharmacological doses.
Genistein is a soy-derived isoflavone and phytoestrogen with antineoplastic activity. Genistein binds to and inhibits protein-tyrosine kinase, thereby disrupting signal transduction and inducing cell differentiation. This agent also inhibits topoisomerase-II, leading to DNA fragmentation and apoptosis, and induces G2/M cell cycle arrest. Genistein exhibits antioxidant, antiangiogenic, and immunosuppressive activities. In adult animals, dietary genistein has chemopreventive effects on breast, prostate, and other endocrine-dependent tumors.
Pharmacological effects
Genistein is a kind of polyphenols compound which can be found in soy and red clover and other plants with its molecular structure being similar to 17β-estradiol and having antioxidant activity and high affinity towards estrogen receptor. It can inhibit the activity of protein tyrosine kinase (PTK) and topoisomerase enzyme activity II with capability of inducing programmed cell death and improve the efficacy of anti-cancer drug as well as inhibit angiogenesis, and so on. It is one type of flavonoids (also called isoflavones) and is often found together with isoflavones called the isoflavones. They are all called soy isoflavones. Those benefits of these kinds of compounds on human health have been studied extensively and it is a promising cancer chemopreventive agent with significant impact on preventing cancer and other diseases. Genistein is the major phytoestrogens in the present study.
Plant Source: legume Genista (broom).
Clinical Applications: it can be used for female beauty care and prevention of blood diseases and cancer.
Genistein contains polyphenol structure with the hydrogen atoms in the phenolic hydroxyl group being prone to dissociate from external oxygen atom from the external interaction, leading to the formation of hydrogen ions to play reduction effect. This is the structure basis for the capability of the genistein of anti-oxidative and reductive. So those substances in food can fight against superoxide anion radicals, blocking the chain reaction of free radicals and play a role in anti-oxidation.
Genistein is not a hormone, but because it can bind to estrogen receptors and plays a weak estrogenic effect, it is called phytoestrogens. Because the activity of isoflavones is only 1/1000 of the estradiol which can competitively bind with estrogen receptors, exhibiting two-way adjustment with the resulting estrogenic effects of much lower harmful effects than estradiol and further with protective effect on then hormone related diseases such as menopause, osteoporosis, elevated blood lipids, etc; for patients of high levels of estrogen, it exhibits anti-estrogenic activity and can prevent breast cancer, endometriosis with two-way balance adjustment function.
Genistein, for the rat high cholesterol induced by TNT (trinitrotoluene) WR1339, has effect of lowering serum cholesterol and triglycerides with the effect on the later one being particularly significant.
This information is edited by Xiongfeng Dai from Chemicalbook.
Physical and Chemical Properties
It appears as pale yellow dendritic needle-like powder with the melting point being 297 ℃-298 ℃; It is soluble in common organic solvents but almost insoluble in water. When being dissolved in dilute alkali, it will become yellow color.
Uses
It has anti-tumor, anti-fungal, blood lipid-lowering and can fight against estrogen activity.
Mechanism of action
Genistein may inhibit cancer cell growth by blocking enzymes required for cell growth. Genistein may decrease cardiovascular risk in postmenopausal women by interacting with the nuclear estrogen receptors to alter the transcription of cell specific genes. In randomized clinical trials, genistein was seen to increase the ratio of nitric oxide to endothelin and improved flow-mediated endothelium dependent vasodilation in healthy postmenopausal women. In addition, genistein may have beneficial effects on glucose metabolism by inhibiting islet tyrosine kinase activity as well as insulin release dependent on glucose and sulfonylurea.
References
https://www.drugbank.ca/drugs/DB01645
Health Benefits
Estrogenic effect
The estrogenic activity of genistein has been confirmed in many studies. Of all the isoflavones, genistein has the strongest estrogenic activity. The estrogenic effect of genistein may explain its protective action against osteoporosis and its possible effect on body weight reductions. Genistein is also used to ease menopause symptoms, such as hot flushes.
Antioxidant
Genistein is a strong antioxidant. Genistein removes damaging free radicals and reduces lipid peroxidation. Genistein increases the activity of other antioxidant enzymes such as glutathione peroxidase, superoxide dismutase and glutathione reductase. Studies have shown that genistein can also influence the growth of cells which are not hormone-dependent.
Anticancer
Genistein seems to reduce the risk for some hormone related cancers, principally breast cancer and prostate cancer. Epidemiological studies show that consumption of isoflavones may protect against breast and prostate cancer. High dietary intake of soy products China and Japan are linked with low incidence of these cancers. There are lots of theories to explain the anti-cancer action of genistein: inhibition of angiogenesis, inhibition of tyrosine kinases, antioxidant property, and anti-estrogen action (it is known that estrogen increases risk for certain cancers). Genistein binds with estrogen receptors, preventing the estrogen from binding and initiating cancer growth.
Heart health
Many in-vitro tests have demonstrated that genistein inhibits cellular cholesterol synthesis and cholesterol esterification. Genistein also reduces fatty acid oxidation and exerts lipid lowering effect. Only oxidized LDL cholesterol is absorbed by the arterial cells and prevention of this oxidation will reduce the risk for arteriosclerosis. Gensistein prevents the formation of hearth attacks and strokes by acting as anticlotting agent.
References
http://www.phytochemicals.info/phytochemicals/genistein.php
Description
Genistein (446-72-0) is a naturally occurring flavonoid with a wide range of biological actions. Inhibits protein tyrosine kinases including epidermal growth factor receptor kinase.1,2?Phytoestrogen3?and agonist at GPR304. Displays cancer chemopreventive activity.5
Chemical Properties
Yellow Crystalline Solid
Uses
Exhibits specific inhibitory activity against tyrosine kinases,including autophosphorylation of epidermal growth factor receptor kinase (IC50 - 2.6uM). Also inhibits other protein kinases through competitive inhibition of ATP. Inhibits tumor cell proliferation and induces tumor cell differentiation. Produces cell-cycle arrest and apoptosis in Jurat T-leukemia cells. However, it prevents anti-CD3 monoclonal antibody-induced thymic apoptosis. Genistein also inhibits topoisomerase II activity in vitro. Genistein has also been shown to inhibit the action of GABA on recombinant GABAA receptors 2. uv(max)ethanol: 262.5 nm (e= 138). moderately sol. in hot alcohol
Uses
cytotoxic inhibitor of tyrosine kinase and topoisomerase II kinase
Uses
genistein is an isoflavone commonly found in soy. It has demonstrated uV-protection properties through anti-oxidant activity. Studies indicate genistein can promote collagen synthesis, making it applicable in anti-aging cosmetics.
Uses
Genistein, a phytoestrogen found in soy products, is a highly specific inhibitor of protein tyrosine kinase (PTK) which blocks the mitogenic effect mediated by EGF on NIH-3T3 cells with IC50 of 12μM or by insulin with IC50 of 19 μM.
Definition
ChEBI: 7-Hydroxyisoflavone with additional hydroxy groups at positions 5 and 4'. It is a phytoestrogenic isoflavone with antioxidant properties.
General Description
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
Biological Activity
Phytoestrogen with a wide range of biological actions. Inhibits protein tyrosine kinases including epidermal growth factor receptor kinase. Also binds to PPAR γ and estrogen receptors and acts as an agonist at GPR30. Also available as part of the MAPK Cascade Inhibitor Tocriset™ .
Biochem/physiol Actions
Inhibitor of tyrosine protein kinase; competitive inhibitor of ATP in other protein kinase reactions. Antiangiogenic agent, down-regulates the transcription of genes involved in controlling angiogenesis.
Mechanism of action
Genistein, an isoflavone isolated from soybeans, exhibits anticarcinogenic and antioxidant properties. Particularly, genistein has been shown to inhibit production of IL-6 and MAPK. Modulation to these cellular events may help regulate and attenuate UVB-induced inflammatory damage to the skin. Moreover, genistein inhibits UV-induced oxidative DNA damage and blocks UV-induced expression of c-fos and c-jun proto-oncogenes.
Anticancer Research
It is an isoflavone and is obtained from a variety of plants like psoralea (Psoraleacorylifolia), kudzu (Pueraria lobata), faba beans (Vicia faba), and soybeans(Glycine max). It exhibits anticancer effect by inhibiting NF-κB and protein kinaseB (Akt) signaling pathways (Singh et al. 2016b). It blocks the proliferation of cancercells via the inhibition of cell growth enzymes and survival like tyrosine kinase andtopoisomerase II; hence it is used to treat leukemia. Genistein increases the growthrate of some estrogen receptors in breast cancer cells and the rate of proliferation of estrogen-dependent breast cancer by competitive binding to the estrogen-β receptors.It may be involved in JNK pathway in inducing activator protein-1(AP-1) activity(Wang et al. 2012; Dixon and Ferreira 2002).
Safety Profile
Experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes.
storage
-20°C (desiccate)
Purification Methods
Crystallise it from EtOH or aqueous EtOH. It has UV: max at 290nm (EtOH). The S(-)-enantiomer (natural form) has m 255-256o (from EtOH) and [] D 20 -28.0o (c 2, EtOH), [ ] D 20 -35.2o (c 1, pyridine).[Beilstein 18 H 503, 18 II 164, 18 III/IV 2630.] Genistein (4',5,7-trihydroxyisoflavone) [446-72-0]M 270.2 crystallises from 60% aqueous EtOH or water with m 297-298o and [] D 20 -28o (c 0.6, 20mM NaOH). [Beilstein 18/4 V 594.]For Naringin (naringenin 7-rhamnoglucoside) See “Carbohydrates” in Chapter 6.
References
1) Akiyama?et al.?(1987),?Genistein, a specific inhibitor of tyrosine-specific protein kinases; J. Biol. Chem.,?262?5592 2) Linassier?et al.?(1990),?Mechanisms of action in NIH-3T3 cells of genistein, an inhibitor if EGF receptor tyrosine kinase?Biochem. Pharmacol.,?39?187 3) Dang?et al.?(2003),?Peroxisome proliferator-activated receptor gamma (PPARgamma) as a molecular target for the soy phytoestrogen genistein; J. Biol. Chem.,?278?962 4) Vivacqua?et al.?(2006),?17beta-estradiol, genistein, and 4-hydroxytamoxifen induce the proliferation of thyroid cancer cells through the g protein-coupled receptor GPR30; Mol. Pharmacol., 70?1414 5) Sarker and Li (2002),?Mechanisms of cancer chemoprevention by soy isoflavone genistein; Cancer Metastasis Rev.,?21?265
Genistein Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Chengdu ChenLv Herb Co.,Ltd | +undefined13608205856 | maryextract@126.com | China | 127 | 58 |
| Bonerge(Hunan) Lifescience Co., Ltd. | +86-731-82791134 +86-18801900056 | hank.h@bonerge.com | China | 30 | 58 |
| Wuhan Quanjinci New Material Co.,Ltd. | +8615271838296 | kyra@quanjinci.com | China | 1532 | 58 |
| Chongqing Zhihe Biopharmaceutical Co., Ltd. | +86-18580541567 +86-17782035140 | sales@zhswyy.com | China | 338 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7845 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3012 | 60 |
View Lastest Price from Genistein manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-26 | Genistein
446-72-0
|
US $0.00-0.00 / kg | 0.10000000149011612kg | ≥98%HPLC | 20tons | Chongqing Zhihe Biopharmaceutical Co., Ltd. | |
 |
2024-04-23 | Genistein powder 50-98%Sophora Japonica Extract
446-72-0
|
US $0.00-0.00 / kg | 1kg | 50-98% | 100000 | Changsha Staherb Natural Ingredients Co., Ltd. | |
 |
2024-04-23 | Genistein Sophora Japonica Extract
446-72-0
|
US $0.00-0.00 / kg | 1kg | 98% | 100000 | Changsha Staherb Natural Ingredients Co., Ltd. |
-

- Genistein
446-72-0
- US $0.00-0.00 / kg
- ≥98%HPLC
- Chongqing Zhihe Biopharmaceutical Co., Ltd.
-

- Genistein powder 50-98%Sophora Japonica Extract
446-72-0
- US $0.00-0.00 / kg
- 50-98%
- Changsha Staherb Natural Ingredients Co., Ltd.
-

- Genistein Sophora Japonica Extract
446-72-0
- US $0.00-0.00 / kg
- 98%
- Changsha Staherb Natural Ingredients Co., Ltd.