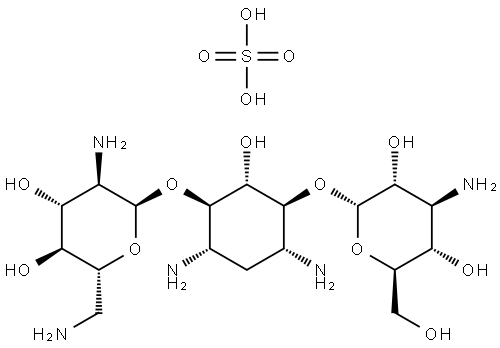
KANAMYCIN B SULFATE
- CAS No.
- 29701-07-3
- Chemical Name:
- KANAMYCIN B SULFATE
- Synonyms
- Kanendos;Stereocidin;BEKANAMYCIN SULFATE;KANAMYCIN B SULFATE;Aminodeoxybekanamycin;Kanamycin B sulfate CRS;BEKANAMYCIN SULFATE SALT;KANAMYCIN B SULFATE SALT;aminodeoxykanamycinsulfate;Kanamycin B Sulfate, EvoPure
- CBNumber:
- CB6761792
- Molecular Formula:
- C18H39N5O14S
- Molecular Weight:
- 581.59
- MDL Number:
- MFCD00078511
- MOL File:
- 29701-07-3.mol
| storage temp. | 2-8°C |
|---|---|
| solubility | H2O: 50 mg/mL, clear, colorless |
| form | powder |
| Merck | 13,5299 |
| BRN | 5235274 |
| Stability | Hygroscopic |
| FDA UNII | KB71EA86HM |
SAFETY
Risk and Safety Statements
| Safety Statements | 22-24/25 |
|---|---|
| WGK Germany | 2 |
| RTECS | WK1976500 |
KANAMYCIN B SULFATE price More Price(7)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | B5264 | Kanamycin B sulfate salt aminoglycoside antibiotic | 29701-07-3 | 100mg | $104 | 2024-03-01 | Buy |
| Sigma-Aldrich | B5264 | Kanamycin B sulfate salt aminoglycoside antibiotic | 29701-07-3 | 1g | $509 | 2024-03-01 | Buy |
| Sigma-Aldrich | B5264 | Kanamycin B sulfate salt aminoglycoside antibiotic | 29701-07-3 | 250mg | $176 | 2024-03-01 | Buy |
| Sigma-Aldrich | K0100000 | Kanamycin B sulfate salt European Pharmacopoeia (EP) Reference Standard | 29701-07-3 | k0100000 | $220 | 2024-03-01 | Buy |
| Usbiological | 015282 | Kanamycin B Sulfate | 29701-07-3 | 25mg | $446 | 2021-12-16 | Buy |
KANAMYCIN B SULFATE Chemical Properties,Uses,Production
Originator
Kanendomycin,Meiji Seika,Japan,1969
Uses
Antibiotic complex produced by Streptomyces kanamyceticus Okami & Umezawa from Japanese soil. Comprised of three components, kanamycin A, the major component, and kanamycins B and C, two minor congeners. Antibacterial.
Definition
ChEBI: Bekanamycin sulfate is an aminoglycoside sulfate salt. It is functionally related to a kanamycin B.
Manufacturing Process
200 liters of the medium containing 2.0% starch, 1.0% soybean meal, 0.05%
KCl, 0.05% MgSO4·7H2O, 0.3% NaCl, 0.2% NaNO3 was placed in the 400 liter
fermenter, the pH was adjusted to 7.5, and the medium was then sterilized
(pH after the sterilization was 7.0) for 30 minutes at 120°C, inoculated with
1,000 ml of 40 hour shake-cultured broth of S. kanamyceticus (a selected
subculture of K2-J strain) and tank-cultured at 27°-29°C. As antifoam,soybean oil (0.04%)and silicone (0.04%) were added. The broth after 48
hours was found to contain 250 mcg/ml of kanamycin.
A portion (950 ml) of the rich eluate was adjusted to pH 6.0 by the addition of
sulfuric acid. Ultrawet K (7.0 g) in 70 ml water was added slowly to the
neutralized eluate to precipitate kanamycin B dodecylbenzenesulfonate which
was collected by filtration after adding filter aid (Dicalite). The cake was
washed with water and extracted with 100 ml methanol. After filtering and
washing with methanol, sulfuric acid was added to the filtrate until no more
kanamycin B sulfate precipitated. After addition of an equal volume of acetone
to provide more complete precipitation, the kanamycin B sulfate was collected
by filtration, washed with methanol and dried in vacuo at 50°C.
Therapeutic Function
D-Streptamine, O-3-amino-3-deoxy-α-D-glucopyranosyl-(1- 6)-O-[2,6-diamino-2,6-dideoxy-α-D-glucopyranosyl-(1-4)]-2-deoxy sulfate (1:1)
Purification Methods
A small quantity of kanamycin B (24mg) can be purified on a small Dowex-1 x 2 column (6 x 50mm); the required fraction is evaporated to dryness and the residue crystallised from EtOH containing a small amount of H2O. [Umezawa et al. Bull Chem Soc Jpn 42 537 1969.] It has been crystallised from H2O by dissolving ~1g in H2O (3mL), adding Me2NCHO (3mL) and setting aside at 4o overnight. The needles are collected and dried to constant weight at 130o. It has also been recrystallised from aqueous EtOH. It is slightly soluble in CHCl3 and isoPrOH. [IR: Wakazawa et al. J Antibiot 14A 180, 187 1961, Ito et al. J Antibiot 17 A 189 1964, Beilstein 18 III/IV 7631.]
KANAMYCIN B SULFATE Preparation Products And Raw materials
KANAMYCIN B SULFATE Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Standardpharm Co. Ltd. | 86-714-3992388 | overseasales1@yongstandards.com | United States | 14336 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 15093356674; | laboratory@coreychem.com | China | 30255 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29220 | 58 |
| Alfa Chemistry | +1-5166625404 | Info@alfa-chemistry.com | United States | 21317 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 52927 | 58 |
| Shanghai Boyle Chemical Co., Ltd. | sales@boylechem.com | China | 2923 | 55 | |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 | jkinfo@jkchemical.com | China | 96815 | 76 |
| Supplier | Advantage |
|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 58 |
| Standardpharm Co. Ltd. | 58 |
| BOC Sciences | 58 |
| TargetMol Chemicals Inc. | 58 |
| Career Henan Chemica Co | 58 |
| Dideu Industries Group Limited | 58 |
| Alfa Chemistry | 58 |
| Aladdin Scientific | 58 |
| Shanghai Boyle Chemical Co., Ltd. | 55 |
| J & K SCIENTIFIC LTD. | 76 |
29701-07-3(KANAMYCIN B SULFATE)Related Search:
1of4