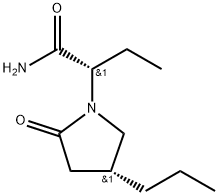
Brivaracetam
- CAS No.
- 357336-20-0
- Chemical Name:
- Brivaracetam
- Synonyms
- Briviact;1-PyrrolidineacetaMide, α-ethyl-2-oxo-4-propyl-, (αS,4R)-;UCB 34714;UCB34714;UCB-34714;(S)-2-((R)-2-oxo-4-propylpyrrolidin-1-yl)butanaMide;(2S)-2-[(4R)-2-oxo-4-propylpyrrolidin-1-yl]butanamide;(alphaS,4R)-alpha-Ethyl-2-oxo-4-propyl-1-pyrrolidineacetamide;Boisitan;Boisetan;Brexitan;UCB-34714
- CBNumber:
- CB81508592
- Molecular Formula:
- C11H20N2O2
- Molecular Weight:
- 212.29
- MDL Number:
- MFCD13152385
- MOL File:
- 357336-20-0.mol
- MSDS File:
- SDS
| Melting point | 76.38° |
|---|---|
| alpha | D25 -60.57° (c = 1 in methanol) |
| Boiling point | 409.3±28.0 °C(Predicted) |
| Density | 1.062 |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 15.74±0.50(Predicted) |
| color | White to Off-White |
| InChI | InChI=1/C11H20N2O2/c1-3-5-8-6-10(14)13(7-8)9(4-2)11(12)15/h8-9H,3-7H2,1-2H3,(H2,12,15)/t8-,9+/s3 |
| InChIKey | MSYKRHVOOPPJKU-MASDURLHNA-N |
| SMILES | [C@H](N1C(C[C@@H](CCC)C1)=O)(CC)C(=O)N |&1:0,4,r| |
| FDA UNII | U863JGG2IA |
| NCI Drug Dictionary | brivaracetam |
| ATC code | N03AX23 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P264-P270-P301+P312-P330-P501 | |||||||||
| NFPA 704 |
|
Brivaracetam price More Price(16)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 23873 | Brivaracetam ≥98% | 357336-20-0 | 10mg | $99 | 2021-12-16 | Buy |
| Cayman Chemical | 23873 | Brivaracetam ≥98% | 357336-20-0 | 25mg | $149 | 2021-12-16 | Buy |
| Cayman Chemical | 23873 | Brivaracetam ≥98% | 357336-20-0 | 50mg | $272 | 2021-12-16 | Buy |
| Cayman Chemical | 23873 | Brivaracetam ≥98% | 357336-20-0 | 100mg | $446 | 2021-12-16 | Buy |
| Tocris | 7271 | Brivaracetam ≥98%(HPLC) | 357336-20-0 | 50 | $545 | 2021-12-16 | Buy |
Brivaracetam Chemical Properties,Uses,Production
Description
Brivaracetam, a chemical analog of Levetiracetam, is a racetam derivative with anticonvulsant effect. It is used for the treatment of partial-onset seizures with or without secondary generalisation, in combination with other antiepileptic drugs. The exact mechanism of brivaracetam's anti-epileptogenic activity is unknown. What is known is thatbrivaracetam binds SV2A with high affinity. SV2A is known to play a role in epileptogenesis through modulation of synaptic GABA release. It is thought that brivaracetam exerts its anti-epileptogenic effects through its binding to SV2A. Brivaracetam can also inhibit Na+ channels which may also contribute to its anti-epileptogenic action.
Overview and History
Brivaracetam is an orally bioavailable levetiracetam derivative, with anticonvulsant activity. It can be used in the treatment of partial-onset seizures[1-3]. Brivaracetam binds SV2A with 20 times higher affinity than levetiracetam. It is available under the brand name Briviact made by UCB. It was approved by the federal drug administration of USA[FDA] on Feb 19th 2016[1-3].
Application
Brivaracetam is indicated for the treatment of partial-onset seizures in patients 4 years of age and older[4,5]. It provides a new monotherapy treatment option for epilepsy patients 16 years of age and older who suffering partial-onset[focal] seizures, which can be initiated at a therapeutic dose at day one[5].
Mode of action
The accurate mode of action of brivaracetam remains unclear. It is known that Brivaracetam can strongly bind to SV2A[6-8], which is known to play a role in epileptogenesis through modulation of synaptic GABA release. It is thought that brivaracetam exerts its anti-epileptogenic effects through its binding to SV2A[6-8]. It may access the luminal side of recycling synaptic vesicles during vesicular endocytosis, which may reduce excitatory neurotransmitter release and enhance synaptic depression during trains of high-frequency activity, such as is believed to occur during epileptic activity. Brivaracetam is also known to inhibit Na+ channels which may also contribute to its anti-epileptogenic action[9].
Adverse reactions
The most frequently reported adverse reactions associated with brivaracetam treatment are somnolence and dizziness (both somnolence and fatigue are reported with a higher incidence with increasing dose). Preliminary metaanalytic data comparing the incidence of behavioural problems in brivaracetam and levetiracetam trials (used as adjunctive therapy in adults with uncontrolled partial- onset epilepsy) showed that the incidence of non- psychotic behavioural treatment emergent adverse effects was considerably lower with brivaracetam treatment (6.8%) compared with levetiracetam (10.9%), whereas the incidences in placebo arms were similar (4.2 and 4.8%, respectively). The placebo- adjusted incidence rates were 2.6% for brivaracetam and 6.8% for levetiracetam, resulting in a brivaracetam/levetiracetam Odds Ratio of 0.68. Brivaracetam has a low interaction potential, although it is not recommended for use with concomitant levetiracetam.
Dose titration
Brivaracetam is known under the proprietary name of Briviact® (UCB Pharma, Slough) in the UK and USA. Brivaracetam is available in different pharmaceutical forms (10-25-50-75-100 mg film- coated tablets and 10 mg/ mL oral solution). Brivaracetam is initially prescribed at the dose of 50–100 mg daily, divided into two doses. Based on individual patient response and tolerability, the dose may be adjusted within the dose range of 50– 200 mg daily. Brivaracetam is recommended as an adjunctive AED treatment for partial- onset seizures with or without secondary generalization. Brivaracetam exhibits greater antiepileptic properties than levetiracetam in animal models, but with a somewhat smaller (although still high) therapeutic range. Moreover, brivaracetam requires no up- titration to reach therapeutic doses and there is no recommendation for specific monitoring. Exposure to brivaracetam is increased in patients with chronic liver disease. A starting dose of 50 mg daily should be considered and a maximum daily dose of 150 mg administered, divided into two doses, is recommended for all stages of hepatic impairment. If patients missed one dose or more, it is recommended that they take a single dose as soon as they remember and take the following dose at the usual morning or evening time, in order to avoid the brivaracetam plasma concentration falling below the efficacy level and prevent breakthrough seizures from occurring. If brivaracetam has to be discontinued, it is recommended to withdraw it gradually by 50 mg daily on a weekly basis. After 1 week of treatment at 50 mg daily, a final week of treatment at the dose of 20 mg daily is recommended.
Warning
It is possible that BRIVIACT can cause the following severe adverse effects, special attention should pay immediately once those following cases occur[4, 10, 11].
Suicidal Behavior and Ideation: Antiepileptic drugs can increase the risk of suicidal behavior and ideation. Monitor patients taking BRIVIACT for the emergence or worsening of depression; unusual changes in mood or behavior; or suicidal thoughts, behavior, or self-harm. Advise patients, their caregivers, and/or families to be alert for these behavioral changes and report them immediately to a healthcare provider[4].
Psychiatric Adverse Reactions: BRIVIACT can also cause psychiatric adverse reactions, including non-psychotic and psychotic symptoms. These events were reported in approximately 13% of patients taking at least 50 mg per day of BRIVIACT compared to 8% of patients taking placebo. A total of 1.7% of adult patients taking BRIVIACT discontinued treatment due to psychiatric reactions compared to 1.3% of patients taking placebo. Advise patients to report these symptoms immediately to a healthcare provider[10].
Hypersensitivity: BRIVIACT can cause hypersensitivity reactions such as bronchospasm and angioedema. The patients should discontinue BRIVIACT if a he/she develops a hypersensitivity reaction after treatment. BRIVIACT should not be applied in patients with a prior hypersensitivity reaction to brivaracetam or any of the inactive ingredients[10].
Neurological Adverse Reactions: BRIVIACT causes somnolence, fatigue, dizziness, and disturbance in coordination. Somnolence and fatigue-related adverse reactions were reported in 25% of patients taking at least 50 mg per day of BRIVIACT compared to 14% of patients taking placebo. Dizziness and disturbance in gait and coordination were reported in 16% of patients taking at least 50 mg per day of BRIVIACT compared to 10% of patients taking placebo. The risk is greatest early in treatment but can occur at any time. Monitor patients for these signs and symptoms and advise them not to drive or operate machinery until they have gained sufficient experience on BRIVIACT[4].
Withdrawal of Antiepileptic Drugs: BRIVIACT should generally be withdrawn gradually because of the risk of increased seizure frequency and status epilepticus[4].
Precaution
You should not use brivaracetam if you are allergic to it[4, 11, 12].
Brivaracetam may become habit-forming for people administrate. Therefore, it is of risk to share brivaracetam with another person, especially avoid sharing with someone with a history of drug abuse or addiction. You should keep the medication in a place where others cannot get to it. Selling or giving away this medicine is against the law.
Before administrate the brivaracetam, tell your doctor if you have ever had one or several of the following symptom for your own safety: Depression or a mood disorder; Suicidal thoughts or actions; liver disease; or alcoholism or drug addiction.
Follow your doctor's instructions about taking seizure medication if you are pregnant. Seizure control is very important during pregnancy, and having a seizure could harm both mother and baby. Do not start or stop taking this medicine without your doctor's advice, and tell your doctor right away if you become pregnant. If you are pregnant, your name may be listed on a pregnancy registry. This is to track the outcome of the pregnancy and to evaluate any effects of brivaracetam on the baby.
It is not known whether brivaracetam passes into breast milk or if it could harm a nursing baby. Tell your doctor if you are breast-feeding a baby.
Brivaracetam is not approved for use by anyone younger than 16 years old[4].
Patients of hepatic impairment should subject to dose adjustment[4].
References
- https://www.ncbi.nlm.nih.gov/pubmed/21575627
- https://www.ucb.com/stories-media/press-releases/article/U-S-FDA-approves-UCB-s-new-epilepsy-treatment-BRIVIACT-for-patients-with-partial-onset-seizures
- https://www.drugbank.ca/drugs/DB05541
- https://www.briviact.com/briviact-PI.pdf
- https://www.ucb.com/stories-media/Press-Releases/article/New-indication-for-BRIVIACT-brivaracetam-UCB-s-newest-antiepileptic-drug-approved-by-FDA-as-monotherapy-treatment-of-partial-onset-seizures-in-adults
- Gillard, Michel, et al. "Binding characteristics of brivaracetam, a selective, high affinity SV2A ligand in rat, mouse and human brain: Relationship to anti-convulsant properties." European Journal of Pharmacology664.1(2011]:36-44.
- Yang, X., et al. "Brivaracetam augments short-term depression and slows vesicle recycling. " Epilepsia 56.12(2016]:1899-1909.
- Nicolas, J. M., et al. "Brivaracetam, a selective high-affinity synaptic vesicle protein 2A[SV2A] ligand with preclinical evidence of high brain permeability and fast onset of action. " Epilepsia 57.2(2016]:201-209.
- Vogl, Christian, et al. "The SV2A Ligand Levetiracetam Inhibits Presynaptic Ca2+ Channels Via an Intracellular Pathway."[2012].
- Briviact® US Prescribing Information. Brussels, Belgium: UCB, 2016.
- https://www.rxlist.com/briviact-side-effects-drug-center.htm
- https://www.everydayhealth.com/drugs/brivaracetam
Description
Brivaracetam, a novel oral antiepileptic drug with a high affinity for synaptic vesicle protein 2A (SV2A), was approved in Europe and the US as an adjunctive therapy for the treatment of partial onset seizures with or without secondary generalization in patients aged 16 or older.42 Brivaracetam is very closely related to levetiracetam, an antiepileptic treatment whose immediate release formulation has been available in the United States as a generic drug since 2008, but whose extended release formulation is under patent protection until 2028. The two drugs, which were both developed by UCB Pharma, are structurally similar with brivaracetam having an n-propyl group at the C-4 position of the pyrrolidinone ring and levetiracetam having a hydrogen at this same position. A systematic investigation of the various substitutions of levetiracetam resulted in the identification of more potent and selective SV2A ligands and ultimately culminated in the discovery of brivaracetam, which has greater affinity for SV2A, improved selectivity, more rapid brain penetration, and faster onset of action against seizures than levetiracetam.
Uses
Treatment ofTreatment of epilepsy, neuropathic pain and essential tremor.
Uses
Brivaracetam, is a 4-n-propyl analog of levetiracetam (L331500), and a racetam derivative with anticonvulsant properties.
Definition
ChEBI: A non-proteinogenic amino acid derivative that is butanamide in which the pro-S hydrogen at position 2 is replaced by a (4R)-2-oxo-4-propylpyrrolidin-1-yl. Used for treatment of partial onset seizures related to epilepsy.
Clinical Use
Antiepileptic agent
Side effects
Common side effects of brivaracetam include: constipation, nausea, vomiting, extreme tiredness or low energy. Serious side effects that may be caused include: swelling of the face, throat, tongue, lips, and eyes; difficulty swallowing or breathing; hoarseness, hallucinations (seeing things or hearing sounds that are not there), and delusions (strange thoughts or beliefs that have no basis in reality). An overdose may cause: drowsiness, extreme tiredness, dizziness, difficulty maintaining balance, blurred or double vision, slowed heartbeat, nausea, and feeling anxious.
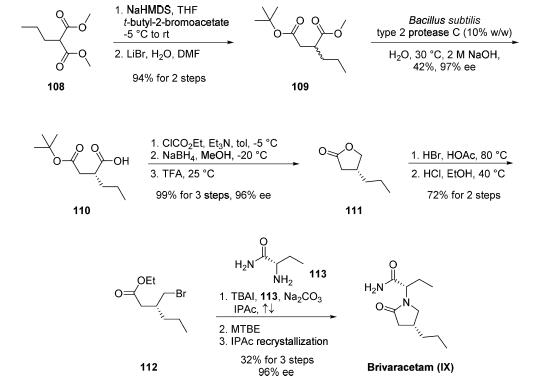
Synthesis
Two enantioselective routes have been reported, one employing
an enzymatic resolution and the other utilizing (R)-
(-)-epichlorohydrin as a chiral starting material. The route involves an enzymatic resolution,
is the only kilogram-scale route disclosed in the literature to
date and reportedly permits the production of brivaracetam
within the required commercial quality specifications. However,
the authors note that the development of this route for
commercial purposes has been stopped. Commercial
dimethyl n-propylmalonate 108 was first alkylated with tertbutyl-
2-bromoacetate. The resulting product underwent
Krapcho decarboxylation to afford racemic succinate derivative
109 in 94% yield over the two steps. Optimized conditions
for the key enzymatic resolution employed protease C from
Bacillus subtilis type 2 at 30 ??C for 18 h to resolve ester 109 and
provide the acid enantiomer 110. This biocatalytic process
allowed for residual unreacted diester 109 to be washed away
with cyclohexane at pH 9 (adjusted with 0.5 M NaOH), and
the desired acid 110 could be isolated upon lowering the pH
(??1) and extracting with isopropyl acetate (42% yield, 97% ee).
The transformation of acid 110 into propyllactone 111
proceeded in nearly quantitative yield by a three-step sequence:
activation of the acid with ethyl chloroformate, reduction to the
alcohol with sodium borohydride, and cyclization upon acidic
workup with TFA. Exposure of 111 to HBr in acetic acid
followed by esterification of the resulting acid-generated
bromoester 112. Finally, TBAI-catalyzed alkylation of 112
with commercial (S)-2-aminobutanamide (113) in refluxing
isopropyl acetate introduced the n-butylamide moiety while
facilitating lactamization. Addition of MTBE followed by filtration and recrystallization from isopropyl acetate afforded
brivaracetam (IX) in 32% yield and 96% ee.
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: concentration reduced by rifampicin.
Antidepressants: antagonism of anticonvulsant effect
(convulsive threshold lowered).
Antimalarials: mefloquine antagonises
anticonvulsant effect.
Antipsychotics: antagonism of anticonvulsant effect
(convulsive threshold lowered).
Orlistat: possibly increased risk of convulsions.
Metabolism
Brivaracetam is mainly metabolised by hydrolysis of the amide moiety to form the corresponding carboxylic acid (approximately 60% the elimination), and secondarily by hydroxylation on the propyl side chain (approximately 30% the elimination). The hydrolysis of the amide moiety leading to the carboxylic acid metabolite (34% of the dose in urine) is supported by hepatic and extra-hepatic amidase. The metabolites are inactive. Greater than 95% of the dose is excreted in the urine as brivaracetam and its metabolites.
storage
Store at -20°C
Brivaracetam Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Beijing Hope Pharmaceutical Co., Ltd. | +86-010-67886402 +8613611125266 | market@hopelife.cn | China | 71 | 58 |
| Chengdu Aupone Pharmaceutical Co.Ltd. | +86-28-+86-28-87843998-6060-6060 +8618631098571 | lijiaqi@aupone.com | China | 54 | 58 |
| Lewoo Pharmatech(Shanghai)Co.,Ltd | +86-15800805830 +86-15800805830 | miao@lewoopharma.com.cn | China | 184 | 58 |
| Biopole Pharmatech Co., Ltd. | +8615151475053 | biopole@163.com | China | 37 | 58 |
| Dorne Chemical Technology co. LTD | +86-86-13583358881 +8618560316533 | Ethan@dornechem.com | China | 3128 | 58 |
| Firsky International Trade (Wuhan) Co., Ltd | +8615387054039 | admin@firsky-cn.com | China | 436 | 58 |
| shandong perfect biotechnology co.ltd | +86-53169958659; +8618596095638 | sales@sdperfect.com | China | 294 | 58 |
| Wuhan Ruichi Technology Co., Ltd | +8613545065237 | admin@whrchem.com | China | 161 | 58 |
| BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD. | +86-18600796368 +86-18600796368 | sales@sjar-tech.com | China | 276 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 18628 | 58 |
Related Qustion
- Q:What is brivaracetam used to treat?
- A:Brivaracetam is an anticonvulsant prescription drug used to treat partial seizures that works by binding to synaptic vesicle g....
- Nov 21,2023
View Lastest Price from Brivaracetam manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-09-26 | Brivaracetam
357336-20-0
|
US $0.00 / Kg/Bag | 2Kg/Bag | 99% up, High Density | 20 tons | Sinoway Industrial co., ltd. | |
 |
2024-09-25 | brivaracetam
357336-20-0
|
US $1.00 / g | 1g | 99% | 100kg | Dorne Chemical Technology co. LTD | |
 |
2024-09-25 | Bri Varacetam
357336-20-0
|
US $0.00 / g | 1g | More Than 99% | 100kg/Month | BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD. |
-

- Brivaracetam
357336-20-0
- US $0.00 / Kg/Bag
- 99% up, High Density
- Sinoway Industrial co., ltd.
-

- brivaracetam
357336-20-0
- US $1.00 / g
- 99%
- Dorne Chemical Technology co. LTD
-

- Bri Varacetam
357336-20-0
- US $0.00 / g
- More Than 99%
- BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD.
357336-20-0( Brivaracetam)Related Search:
1of4