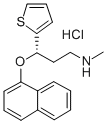
Duloxetine
- CAS No.
- 116539-59-4
- Chemical Name:
- Duloxetine
- Synonyms
- DULOXETIN;DULOXETINE HCI;DULOXETINE-D3;(S)-DULOXETINE;(3S)-N-methyl-3-naphthalen-1-yloxy-3-thiophen-2-yl-propan-1-amine;CS-559;DULOXETINE;(+)-Duloxetine;Duloxetine, >=99%;Duloxetine Hcl(S)
- CBNumber:
- CB8670379
- Molecular Formula:
- C18H19NOS
- Molecular Weight:
- 297.41
- MDL Number:
- MFCD06801358
- MOL File:
- 116539-59-4.mol
- MSDS File:
- SDS
| Boiling point | 466.2±40.0 °C(Predicted) |
|---|---|
| Density | 1.158±0.06 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| pka | 10.02±0.10(Predicted) |
| BCS Class | 2 |
| InChI | InChI=1/C18H19NOS.ClH/c1-19-12-11-17(18-10-5-13-21-18)20-16-9-4-7-14-6-2-3-8-15(14)16;/h2-10,13,17,19H,11-12H2,1H3;1H/t17-;/s3 |
| InChIKey | BFFSMCNJSOPUAY-VOPAOICTNA-N |
| SMILES | C1(=CC=CS1)[C@H](CCNC)OC1=CC=CC2=CC=CC=C12.Cl |&1:5,r| |
| FDA UNII | O5TNM5N07U |
| ATC code | N06AX21 |
| EPA Substance Registry System | 2-Thiophenepropanamine, N-methyl-?-(1-naphthalenyloxy)-, (?S)- (116539-59-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319 |
| Precautionary statements | P501-P270-P264-P280-P302+P352-P337+P313-P305+P351+P338-P362+P364-P332+P313-P301+P312+P330 |
Duloxetine price More Price(13)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Matrix Scientific | 094812 | (S)-N-Methyl-3-(naphthalen-1-yloxy)-3-(thiophen-2-yl)propan-1-amine 95+% | 116539-59-4 | 1g | $135 | 2021-12-16 | Buy |
| Biorbyt Ltd | orb146144 | Duloxetine >98% | 116539-59-4 | 100mg | $414.8 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | RDL0017905 | DULOXETINE-D3 95.00% | 116539-59-4 | 5MG | $504.27 | 2021-12-16 | Buy |
| Biorbyt Ltd | orb146144 | Duloxetine >98% | 116539-59-4 | 250mg | $615.4 | 2021-12-16 | Buy |
| Matrix Scientific | 094812 | (S)-N-Methyl-3-(naphthalen-1-yloxy)-3-(thiophen-2-yl)propan-1-amine 95+% | 116539-59-4 | 5g | $360 | 2021-12-16 | Buy |
Duloxetine Chemical Properties,Uses,Production
Uses
Antidepressant.
Definition
ChEBI: (S)-duloxetine is a duloxetine. It is an enantiomer of a (R)-duloxetine.
brand name
Cymbalta (Lilly).
General Description
Duloxetine (Cymbalta) is a newer antidepressant. It islargely like venlafaxine, which is an SNERI (selective norepinephrinereuptake inhibitor).
Pharmacokinetics
Duloxetine appears to be fairly well absorbed after oral doses, with peak plasma levels in 6 to 10 hours and
linear pharmacokinetics. The drug is extensively metabolized in the liver to active
metabolites, with 72% of an oral dose primarily excreted in the urine as conjugated metabolites and up to
15% appearing in the feces.
N-demethylation to an active metabolite (CYP2D6) and hydroxylation of the naphthyl ring (CYP1A2) at either
the 4-, 5-, or 6-positions are the main metabolic pathways for duloxetine. Its metabolites are primarily
excreted into the urine as glucuronide, sulfate, and O-methylated conjugation products. The
major metabolites found in plasma also were found in the urine. Preclinical data for 4-hydroxyduloxetine
suggests it has a similar pharmacological profile to duloxetine, with selective inhibition of SERT but less
activity at the NET.
Clinical Use
Duloxetine has been approved for the treatment of depression and diabetic peripheral neuropathic pain. It is another analogue in the line of fluoxetine-based products from Lilly, in which the phenyl and phenoxy groups of fluoxetine have been respectively replaced with the benzene isostere, thiophene, and a naphthyloxy group (previously described under fluoxetine). Duloxetine exhibits dual inhibition with high affinity for the SERTs and NETs, with a five times preferential inhibition of the SERT. Duloxetine appears to be a more potent in vitro blocker of SERTs and NETs than venlafaxine. In humans, duloxetine has a low affinity for the other neuroreceptors, suggesting low incidence of unwanted adverse effects.
Synthesis
Reaction of 2-acetylthiophene
with paraformaldehyde and dimethylamine in
ethanol gives 3-(dimethylamino)-1-(2-thienyl)-
1-propanone, which is enantioselectively reduced
with a 2:1 complex of (2R,3S)-4-(dimethylamino)-
3-methyl-1,2-diphenyl-2-butanol
and LiAlH4 in toluene to yield (S)-3-(dimethylamino)-
1-(2-thienyl)-1-propanol. The
condensation of (S)-3-(dimethylamino)-1-(2-
thienyl)-1-propanol with 1-fluoronaphthalene
catalyzed by NaH in DMSO affords the corresponding
naphthyl ether (S)-N,N-dimethyl-3-
(naphthalen-1-yloxy)-3-(thiophen-2-yl)propan-
1-amine, which is finally monodemethylated
with 2,2,2-trichloroethyl chloroformate and zinc
in toluene and treated with oxalic acid .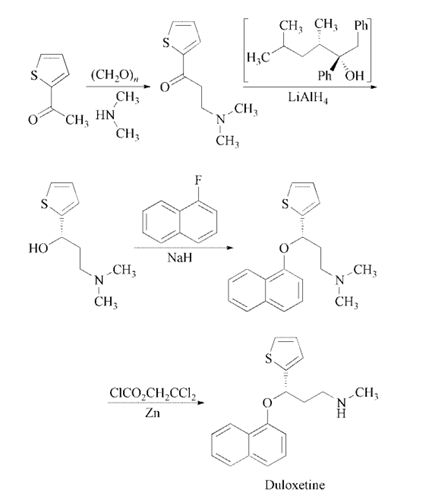
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: metabolism inhibited by ciprofloxacin
- avoid.
Anticoagulants: possibly increased risk of bleeding
with dabigatran.
Other CNS medication: enhanced effect.
Antidepressants: avoid with MAOIs, moclobemide,
St John’s wort, tryptophan, venlaflaxine, amitriptyline,
clomipramine and SSRIs due to increased risk of
serotonin syndrome; increased risk of side effects
with tricyclic antidepressants; fluvoxamine decreases
the clearance of duloxetine by 77% - avoid; possible
increased risk of convulsions with vortioxetine.
Antimalarials: avoid with artemether/lumefantrine
and piperaquine with artenimol.
Dapoxetine: avoid concomitant use.
Methylthioninium: risk of CNS toxicity - avoid if
possible.
Metabolism
Duloxetine is extensively metabolised and the metabolites are excreted principally in urine. Both cytochromes P450-2D6 and 1A2 catalyse the formation of the two major metabolites, glucuronide conjugate of 4-hydroxy duloxetine and sulphate conjugate of 5-hydroxy, 6-methoxy duloxetine. Based upon in vitro studies, the circulating metabolites of duloxetine are considered pharmacologically inactive
Duloxetine Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63711 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | ada@ipurechemical.com | China | 10326 | 58 |
| WinWin Chemical CO., Limited | +86-0577-64498589 +8615325081899 | sales@win-winchemical.com | China | 13854 | 58 |
View Lastest Price from Duloxetine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-03-31 | Duloxetine
116539-59-4
|
US $40.00-20.00 / kg | 1kg | 99% | 2000000 | Shanghai Chinqesen Biotechnology Co., Ltd. | |
 |
2023-01-31 | Raw Powder API Duloxetine
116539-59-4
|
US $70.00 / Kg/Bag | 25Kg/Bag | 99.99% | 200ton | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-01-31 | Duloxetine
116539-59-4
|
US $0.00 / Kg/Drum | 1Kg/Drum | 99% | 5000KG | Hebei Mojin Biotechnology Co., Ltd |
-

- Duloxetine
116539-59-4
- US $40.00-20.00 / kg
- 99%
- Shanghai Chinqesen Biotechnology Co., Ltd.
-

- Raw Powder API Duloxetine
116539-59-4
- US $70.00 / Kg/Bag
- 99.99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Duloxetine
116539-59-4
- US $0.00 / Kg/Drum
- 99%
- Hebei Mojin Biotechnology Co., Ltd
116539-59-4(Duloxetine)Related Search:
1of4