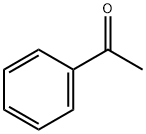
Acetophenone
- CAS No.
- 98-86-2
- Chemical Name:
- Acetophenone
- Synonyms
- 1-Phenylethanone;Acetophenon;Ethanone, 1-phenyl-;Phenylethanone;ACETOPHENONE extrapure AR;HYPNONE;FEMA 2009;1-Phenyl-1-ethanone;METHYL PHENYL KETONE;Acetophenone PG, 99%
- CBNumber:
- CB5694882
- Molecular Formula:
- C8H8O
- Molecular Weight:
- 120.15
- MOL File:
- 98-86-2.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/5/7 12:58:23
| Melting point | 19-20 °C (lit.) |
|---|---|
| Boiling point | 202 °C (lit.) |
| Density | 1.03 g/mL at 25 °C (lit.) |
| vapor density | 4.1 (vs air) |
| vapor pressure | 0.45 mm Hg ( 25 °C) |
| FEMA | 2009 | ACETOPHENONE |
| refractive index |
n |
| Flash point | 180 °F |
| storage temp. | Store below +30°C. |
| solubility | 6.1g/l |
| form | Liquid |
| color | Clear colorless to light yellow |
| Relative polarity | 4.4 |
| Odor | Pungent, floral odor |
| Odor Type | floral |
| biological source | synthetic |
| explosive limit | 1.4-5.2%(V) |
| Water Solubility | 5.5 g/L (20 ºC) |
| Merck | 14,73 |
| JECFA Number | 806 |
| BRN | 605842 |
| Exposure limits | No exposure limits are set. The health hazard from exposure to this compound should be low, due to its low vapor pressure and low toxicity. |
| Dielectric constant | 17.4(25℃) |
| Stability | Stable. Incompatible with strong oxidizing agents, strong bases, strong reducing agents. Combustible. |
| InChIKey | KWOLFJPFCHCOCG-UHFFFAOYSA-N |
| LogP | 1.65 at 20℃ |
| Surface tension | 39.54 mN/m at 283.15K |
| CAS DataBase Reference | 98-86-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetophenone(98-86-2) |
| EPA Substance Registry System | Acetophenone (98-86-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H319 | |||||||||
| Precautionary statements | P301+P312+P330-P305+P351+P338 | |||||||||
| Hazard Codes | Xn,T,F | |||||||||
| Risk Statements | 22-36-63-43-36/37/38-23/24/25-45-39/23/24/25-11-67-40 | |||||||||
| Safety Statements | 26-36/37-24/25-23-53-45-16-7 | |||||||||
| RIDADR | UN 1593 6.1/PG 3 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | AM5250000 | |||||||||
| F | 8 | |||||||||
| Autoignition Temperature | 570 °C | |||||||||
| TSCA | Yes | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29143900 | |||||||||
| Hazardous Substances Data | 98-86-2(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 orally in rats: 0.90 g/kg (Smyth, Carpenter) | |||||||||
| NFPA 704 |
|
Acetophenone price More Price(26)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | W200910 | Acetophenone natural, 98%, FG | 98-86-2 | 1SAMPLE-K | ₹5196 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W200910 | Acetophenone natural, 98%, FG | 98-86-2 | 100G | ₹18835.5 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W200910 | Acetophenone natural, 98%, FG | 98-86-2 | 500G | ₹78091.55 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W200910 | Acetophenone natural, 98%, FG | 98-86-2 | 1KG | ₹149460.78 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W200905 | Acetophenone ≥98%, FG | 98-86-2 | 1SAMPLE-K | ₹5141.88 | 2022-06-14 | Buy |
Acetophenone Chemical Properties,Uses,Production
Description
Acetophenone is the simplest aromatic ketone and is a clear liquid/crystal and very slightly soluble in water with a sweet pungent taste and odour resembling oranges. It is used as a polymerisation catalyst for the manufacture of olefins. Acetophenone is used in perfumery as a fragrance ingredient in soaps, detergents, creams, lotions, and perfumes; as a flavouring agent in foods, non-alcoholic beverages, and tobacco; as a specialty solvent for plastics and resins; as a catalyst for the polymerisation of olefins; and as a photosensitiser in organic syntheses. Acetophenone is a raw material for the synthesis of some pharmaceuticals and is also listed as an approved excipient by the U.S. FDA. Acetophenone occurs naturally in many foods such as apple, apricot, banana, and beef. Acetophenone has been detected in ambient air and drinking water; exposure of the general public may occur through the inhalation of contaminated air or the consumption of contaminated water. It is highly flammable and will get easily ignited by heat, sparks, or flames, and the vapours may form explosive mixtures with air.
Chemical Properties
Acetophenone is a colorless, oily liquid with a sweet, floral odor.It is a naturally occurring component of a large number of foods and essential oils.
Acetophenone can be hydrogenated catalytically to 1-phenylethanol. It is obtained as a by-product in the Hock phenol synthesis and is purified from the high-boiling residue by distillation. The quantities obtained from this source satisfy the present demand.
Acetophenone is used for perfuming detergents and industrial products and is an intermediate in the synthesis of other fragrance materials.
Occurrence
Reported found in cocoa, beef, raspberry, peas, and concord grape
Uses
Acetophenone is a reagent used in the production of fragrances and resin polymers.
Preparation
From benzene and acetylchloride in the presence of aluminum chloride or by catalytic oxidation of ethyl benzene; also prepared by fractional distillation and crystallization from the essential oil of Stirlingia latifolia.
Definition
ChEBI: Acetophenone is a methyl ketone that is acetone in which one of the methyl groups has been replaced by a phenyl group. It has a role as a photosensitizing agent, an animal metabolite and a xenobiotic.
Production Methods
Most methyl phenyl ketone originates from the Hock process for the production of phenol from isopropylbenzene (→Phenol); it is isolated from the residue of this process. In addition, acetophenone can be obtained as a main product by selective decomposition of cumene hydroperoxide in the presence of copper catalysts at 100℃: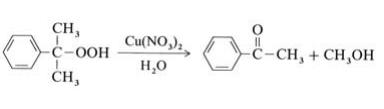
A second possibility is the oxidation of ethylbenzene with air or oxygen at 130℃ and 0.5 MPa. Catalysts used include cobalt salts or manganese salts of naphthenic or fatty acids. Conversion of ethylbenzene is limited to ca. 25 % to minimize the byproducts 1-phenylethanol and benzoic acid. A third method is the Friedel – Crafts acetylation of benzene with acetic anhydride, but this is not of industrial importance.
General Description
Acetophenone appears as a colorless liquid with a sweet pungent taste and odor resembling the odor of oranges. Freezes under cool conditions. Slightly soluble in water and denser than water. Hence sinks in water. Vapor heavier than air. A mild irritant to skin and eyes. Vapors can be narcotic in high concentrations. Used as a flavoring, solvent, and polymerization catalyst.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
Acetophenone reacts with many acids and bases liberating heat and flammable gases (e.g., H2). Reacts with many oxidizing agents. Reacts with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. The amount of heat in these reactions may be sufficient to start a fire in the unreacted portion. Incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides.
Health Hazard
Acetophenone is an irritant, mutagen, and amildly toxic compound. In rabbits 0.77 mgproduced severe eye irritation, but the actionon skin was mild. In mice, subcutaneousadministration of this compound producedsleep; a dose of 330 mg/kg was lethal.
LD50 value, intraperitoneal (mice): 200mg/kg
No symptoms of severe toxicity, nor its carcinogenicityin humans, has been reported..
Fire Hazard
Combustible liquid; flash point (closed cup) 82°C (180°F); vapor pressure 1 torr at 37°C (98.6°F); vapor density 4.1 (air = 1); autoignition temperature 570°C (1058°F); fire-extinguishing agent: dry chemical, foam, or CO2; water may cause frothing, but it can be used to flush and dilute the spill. Its reaction with strong oxidizers may be violent.
Safety Profile
Poison by intraperitoneal and subcutaneous routesModerately toxic by ingestion. A skin and severe eye irritant. Mutation data reported. Narcotic in high concentration. A hypnotic. Flammable liquid. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and fumes. See also IGTONES
Potential Exposure
Acetophenone is used as a solvent and in perfume manufacture to impact a pleasant jasmine or orange-blossom odor. It is used as a catalyst in olefin polymerization and as a flavorant in tobacco. It is also used in the synthesis of pharmaceuticals
Carcinogenicity
No carcinogenicity studies were identified for acetophenone. The U.S. EPA has classified acetophenone as a Category D, not classifiable as to human carcinogenicity.
Environmental Fate
It is unclear what mechanism is responsible for the central nervous system depression observed following high doses of acetophenone. In vitro evaluations have demonstrated that acetophenone suppresses voltage-gated ion channels in olfactory receptor cells and retinal neurons; however, it is unclear if this is related to any of the observed toxicity in animal studies.
Metabolism
At one time, acetophenone was used as a hypnotic. Its conversion to benzoic acid and methylphenylcarbinol in dogs and rabbits was observed by a number of early workers. Small amounts are also excreted as mandelic acid. In the rabbit about half the dose is excreted as methylphenylcarbinyl glucuronide and about 20 % as hippuric acid. It is probable that the ketone is first asymmetrically reduced to the carbinol, which is the precursor of benzoic and mandelic acids.
Solubility in organics
miscible with ethyl alcohol, essential oils and perfume chemicals. Sp.Gr. = 1.033.
Shipping
UN1993 Flammable liquids, n.o.s., Hazard Class: 3; Labels: 3-Flammable liquid, Technical Name Required.
Purification Methods
Dry it by fractional distillation or by standing with anhydrous CaSO4 or CaCl2 for several days, followed by fractional distillation under reduced pressure (from P2O5, optional), and careful, slow and repeated partial crystallisations from the liquid at 0o excluding light and moisture. It can also be crystallised at low temperatures from isopentane. Distillation can be followed by purification using gas-liquid chromatography [Earls & Jones J Chem Soc, Faraday Trans 1 71 2186 1975.] [Beilstein 7 H 271, 7 IV 619.] § A commercial polystyrene supported version is available — scavenger resin (for diol substrates).
Incompatibilities
May form explosive mixture with air. See flash point, above. Reacts violently with strong oxidizers, many acids, bases, amines, amides, and inorganic hydroxides; alkali metals; hydrides, and nitrides. Reacts with reducing agents; alkali metals; hydrides, nitrides. Contact with all preceding materials release heat and flammable gases, including hydrogen; the heat may be sufficient enough to result in fire. Incompatible with aldehydes, aliphatic amines, alkanolamines, cyanides, isocyanates, organic acids, peroxides; perchloric acid. May attack plastics, and some rubbers and coatings
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration, preferably with a flammable solvent
Acetophenone Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Nagar Haveli Perfumes & Aromatics | +917506551149 | Mumbai, India | 230 | 58 | Inquiry |
| Sudarshan Pharma Industries Limited | +91-2242221111 +91-9320932107 | Maharashtra, India | 37 | 58 | Inquiry |
| Anant Pharmaceuticals Pvt Ltd | +91-8550986868 +91-9485998001 | Haryana, India | 460 | 58 | Inquiry |
| ADVENT CHEMBIO PRIVATE LIMITED | +91-9821524116 +91-9821524116 | Mumbai, India | 353 | 58 | Inquiry |
| Soham Chemical Industries | +91-7016081644 +91-7016081644 | Mumbai, India | 82 | 58 | Inquiry |
| PERFECT CHEMICAL | +91-9820465461 +91-9820465461 | Mumbai, India | 391 | 58 | Inquiry |
| SHYAMAL LIFE SCIENCE PVT LTD | +91-9824420566 +91-9824420566 | Gujarat, India | 12 | 58 | Inquiry |
| SI Group India Limited | +91-2356660401 +91-2356660401 | Maharashtra, India | 32 | 58 | Inquiry |
| S D Fine Chem Limited | +91-9323715856 +91-9323715856 | Maharashtra, India | 368 | 58 | Inquiry |
| Real Chemsys Products Pvt Limited | +91-1204216880 +91-1204212000 | New Delhi, India | 29 | 58 | Inquiry |
Related articles
- Acetophenone: Applications, Pharmacokinetics and Synthesis
- Acetophenone is an organic compound with the chemical formula C8H8O. It is a colorless liquid with a sweet, flowery odor that ....
- Apr 26,2023
- What is Acetophenone?
- Acetophenone is an aromatic ketone found naturally in fruits, berries, nuts, and meat. Charles Friedel is credited with the fi....
- Oct 15,2021
- Acetophenone–Chemical Properties and Reactivity
- Acetophenone is a colorless crystal, or light yellow oily liquid, and has many unique chemical properties. It has been reporte....
- Apr 1,2020
Related Qustion
- Q:What are the uses and hazards of Acetophenone?
- A:Acetophenone is an important organic reagent widely used in the food, flavouring, chemical, pharmaceutical and plastics indust....
- Apr 26,2024





