酢酸ビニル (モノマー) 化学特性,用途語,生産方法
外観
無色澄明の液体
定義
本品は、次の化学式で表される不飽和エステルである。
性質
酢酸ビニルの融点は−100.2°Cで、沸点は72〜73°Cです。光や熱によって容易に重合して、ポリ酢酸ビニルが生成します。そのため、 (英: hydroquinone) のような重合禁止剤が微量に含まれています。
実験で用いる場合には、重合禁止剤を除去するため、精製が必要です。酢酸ビニルを希酸やアルカリで加水分解すると、酢酸とアセトアルデヒドが生じます。酢酸ビニルは紫外線で分解して、ケトン、アルデヒド、アルコールなどが生成します。
酢酸ビニルは、酢酸とビニルアルコールから構成されるエステルです。分子式はC4H6O2と表されます。分子量は86.09で、比重は0.9312です。
なお、国際がん研究機関によると、酢酸ビニルは「ヒトへの発がん性が疑われる物質」に分類されています。10,000ppmでマウスに食道がんが見られたと報告例があります。ただし、ヒトに対して発がん性が現れる証拠は得られていません。
溶解性
水に微溶 (2.5g/100ml, 20℃)。エタノール、アセトン、酢酸エチル及びベンゼンに極めて溶けやすく、水にほとんど溶けない。
解説
C4H6O2(86.09).CH2=CHOCOCH3.工業的には,かつては水銀(Ⅱ)触媒を用いてアセチレンと酢酸との反応で合成されていたが,現在ではすべて酸素の共存下でエテンと酢酸との反応で製造されている.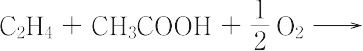 "CH2=CHOCOCH3 + H2O パラジウム触媒を用いる気相法(100~200 ℃)と塩化パラジウム触媒を用いる液相法とがあるが,気相法が主力である.甘い特異臭のある無色の液体.融点-93 ℃,沸点73 ℃.d204 0.932.n20D 1.3950.エタノール,エーテルに可溶.ポリ(酢酸ビニル)(酢酸ビニル樹脂)の単量体で,合成繊維ビニロンの中間原料として用いられる.塗料、接着剤、製紙用サイズ、チューインガムのベースなどに使われている。ポリビニルアルコールを合成する原料として重要である。森北出版「化学辞典(第2版)
"CH2=CHOCOCH3 + H2O パラジウム触媒を用いる気相法(100~200 ℃)と塩化パラジウム触媒を用いる液相法とがあるが,気相法が主力である.甘い特異臭のある無色の液体.融点-93 ℃,沸点73 ℃.d204 0.932.n20D 1.3950.エタノール,エーテルに可溶.ポリ(酢酸ビニル)(酢酸ビニル樹脂)の単量体で,合成繊維ビニロンの中間原料として用いられる.塗料、接着剤、製紙用サイズ、チューインガムのベースなどに使われている。ポリビニルアルコールを合成する原料として重要である。森北出版「化学辞典(第2版)
用途
酢酸ビニルモノマー(VAM)は、合成原料、各種共重合原料、接着剤原料として広範囲にわたって使用されています。
用途
ラッカー、塗料、接着剤、チューイングガム、酢酸ビニル樹脂の合成原料、織物仕上げ、ペーパーコーティング、のためのプラスチックフィルム(食品包装)、重合調整剤(食品用でんぷん用)
製造法
以前は、亜鉛や水銀などの金属塩を触媒としてアセチレンに酢酸を付加させる方法により工業的に製造されていたが、現在ではアセチレンを原料とする方法はコストが高くつくのでほとんど行われなくなり、1960年代から酢酸とエチレンを原料とする製造法が主流になっている。この方法では、酢酸パラジウムなどの触媒を用いて、酸素によるエチレンの酸化と酢酸によるエステル化を1段階の反応で行い、95%以上の高い収率で酢酸ビニルを合成している。
化粧品の成分用途
皮膜形成剤
合成法
工業的に酢酸ビニルは、パラジウム触媒の存在下で、エチレンと酢酸の酸素との反応によって合成されます。この反応はワッカー酸化 (英: Wacker oxidation) と呼ばれています。
酢酸ビニルは金属触媒の存在下で、アセチレン (英: acetylene) に酢酸を付加して製造可能です。1912年に水銀 (II) 触媒を使用して、フリッツ・クラッテ (英: Fritz Klatte) によって合成されました。現在では、触媒に酢酸亜鉛が選択されています。
それ以外にも、エチリデンジアセテート (英: ethylidene diacetate) の熱分解によって、酢酸ビニルが生成します。
主な用途/役割
塩化ビニル樹脂系接着剤の架橋剤として使用される。
説明
Vinyl acetate monomer (VAM) is a colourless liquid, immiscible or slightly soluble in water.
VAM is a flammable liquid. VAM has a sweet, fruity smell (in small quantities), with sharp,
irritating odour at higher levels. VAM is an essential chemical building block used in a wide
variety of industrial and consumer products. VAM is a key ingredient in emulsion polymers,
resins, and intermediates used in paints, adhesives, coatings, textiles, wire and cable polyethylene
compounds, laminated safety glass, packaging, automotive plastic fuel tanks, and
acrylic fibres. Vinyl acetate is used to produce polyvinyl acetate emulsions and resins. Very
small residual levels of vinyl acetate have been found present in products manufactured
using VAM, such as moulded plastic items, adhesives, paints, food packaging containers,
and hairspray.
化学的特性
Vinyl acetate is a colorless, flammable liquid with a pungent odor. The odor threshold is 0.12 ppm 0.3 ppm (NY, NJ).it is the precursor to polyvinyl acetate, an important polymer in industry.
物理的性質
Colorless, watery liquid with a pleasant, fruity odor. Experimentally determined detection and
recognition odor threshold concentrations were 400 μg/m
3 (120 ppb
v) and 1.4 mg/m
3 (400 ppb
v),
respectively (Hellman and Small, 1974).
使用
Vinyl acetate is primarily used to produce polyvinyl acetate
emulsions and polyvinyl alcohol. The principal use of these
emulsions has been in adhesives, paints, textiles, and paper
products.
製造方法
The major industrial route involves the reaction of ethylene and acetic acid with oxygen in the presence of a palladium catalyst.
Ethylene + acetic acid + 1/2 O
2 → Vinyl acetate +H
2O
But by products are also generated:
Ethylene + 3 O
2 → 2CO
2 + 2H
2O
Vinyl acetate is also prepared by the gas-phase addition of acetic acid to acetylene.
調製方法
Vinyl acetate is an industrial chemical that is produced in large amounts in the United States. The worldwide production capacity of vinyl acetate monomer (VAM) was estimated at 6,154,000 tonnes/annum in 2007, with most capacity concentrated in the United States (1,585,000 all in Texas), China (1,261,000), Japan (725,000) and Taiwan (650,000) . The average list price for 2008 was $1600/tonne. Celanese is the largest producer (ca 25% of the worldwide capacity), while other significant producers include China Petrochemical Corporation (7 %), Chang Chun Group (6%) and LyondellBasell (5%).
It is a key ingredient in furniture-glue.
反応性
Vinyl acetate undergoes many of the reactions anticipated for an alkene and an ester. Bromine adds to give the dibromide. Hydrogen halides add to give 1-haloethyl acetates, which cannot be generated by other methods because of the non - availability of the corresponding halo-alcohols. Acetic acid adds in the presence of palladium catalysts to give ethylidene diacetate, CH
3CH(OAc)
2. It undergoes transesterification with a variety of carboxylic acids.The alkene also undergoes Diels - Alder and 2 + 2 cycloadditions.
一般的な説明
Vinyl acetate appears as a clear colorless liquid. Flash point 18 °F. Density 7.8 lb / gal. Slightly soluble in water. Vapors are heavier than air. Vapors irritate the eyes and respiratory system. May polymerize if heated or contaminated. If polymerization occurs inside a container, the container may violently rupture. Used to make adhesives, paints, and plastics.
空気と水の反応
Highly flammable. Slightly soluble in water.
反応プロフィール
Vinyl acetate may undergo spontaneous exothermic polymerization on exposure to light. Reacts with air or water to produces peroxides that initiate explosively violent polymerization. Reacts with hydrogen peroxide to form explosive peracetic acid. Reacts with oxygen to form explosive peroxides. Forms explosive Vinyl acetate ozonide on contact with ozone. Undergoes violent or explosive reactions with 2-aminoethanol, chlorosulfonic acid, ethylenediamine, mineral acids (hydrochloric acid, hydrofluoric acid, nitric acid, sulfuric acid, oleum), and peroxides [Lewis, 3rd ed., 1993, p. 1311]. Polymerization initiated by dibenzoyl peroxide in ethyl acetate accelerated out of control, ignited and exploded [Vervalin, 1973, p. 81]. Polymerization in toluene solution has caused several large industrial explosions [MCA Case History No. 2087].
健康ハザード
Vinyl acetate has been related to reproductive abnormalities. It is a skin and upper respiratory tract irritantand a central nervous system depressant. Exposure caused gradual deterioration of heart muscles.
火災危険
When heated to decomposition, Vinyl acetate burns and emits acrid fumes. Highly dangerous when exposed to heat, flames or oxidizers; explosion hazard with strong acids and strong oxidizers. Incompatible with alumina, oxidizing materials, 2-aminoethanol, chlorosulfonic acid; ethyleneimine; 36% hydrochloric acid; 48.7% hydrofluoric acid; 70% nitric acid; oleum; 96% sulfuric acid; ethylene diamine; peroxides and silica gel. Avoid light or any polymerizing initiator. Hazardous polymerization can be initiated by organic and inorganic peroxides; azo compounds; redox systems (including organometallic components); light; and high energy radiation.
使用用途
酢酸ビニルは、主にとポリビニルアルコールを製造するために使用されます。これらのポリマーは、さまざまな工業製品やコンシューマー製品で使用可能です。
ポリ酢酸ビニルは、塗料、、人工芝、義歯安定剤、ガムペースト、ワイヤー、自動車用プラスチック燃料タンク、食品包装容器等に幅広く利用されています。
その一方で、ポリビニルアルコールは、液晶ディスプレイの偏光板原料、繊維加工、紙加工剤、乳化分散剤、セラミックバインダー等に利用可能です。
安全性プロファイル
Confirmed carcinogen
with experimental carcinogenic and
tumorigenic data. Moderately toxic by
ingestion, inhalation, and intraperitoneal
routes. A skin and eye irritant. Experimental
reproductive effects. Human mutation data
reported. Highly dangerous fire hazard when
exposed to heat, flame, or oxidzers. A
storage hazard, it may undergo spontaneous
exothermic polymerization. Reaction with
air or water to form peroxides that catalyze
an exothermic polymerization reaction has
caused several large industrial explosions.
Reaction with hydrogen peroxide forms the
explosive peracetic acid. Reacts with oxygen
above 50℃ to form an unstable explosive
peroxide. Reacts with ozone to form the
explosive vinyl acetate ozonide. Solution
polymerization of the acetate dmolved in
toluene has resulted in large industrial
explosions. Polymerization reaction with
dibenzoyl peroxide + ethyl acetate may
release ignitable and explosive vapors. The
vapor may react vigorously with desiccants
(e.g., sihca gel or alumina). Incompatible
(explosive) with 2-amino ethanol,
chlorosulfonic acid, ethylenediamine,
ethyleneimine, HCl, HF, HNO3, oleum,
peroxides, H2SO4. See also ESTERS.
職業ばく露
Vinyl acetate is used primarily in
polymerization processes to produce polyvinyl acetate;
polyvinyl alcohol, and vinyl acetate copolymer. The polymers,
usually made as emulsions, suspensions, solutions, or
resins, are used to prepare adhesives, paints, paper coatings,
and textile finishes. Low molecular weight vinyl acetate is
used as a chewing gum base.
概要
酢酸ビニルとは、ビニルアルコールとのエステルです。
ビニルアセタート (英: vinyl acetate) とも呼ばれています。無色透明の可燃性液体です。甘い香りやかすかな刺激臭があります。有機合成原料、PVA (ポバール、) 、塗料などに利用可能です。
酢酸ビニルの製法は1912年に発見されたアセチレン法から始まり、エチレン法、エチリデンジアセタート法と推移しています。なお、酢酸ビニルモノマーの生産拠点は、米国、中国、日本、台湾に集中しています。
発がん性
There is inadequate evidence
in humans for the carcinogenicity of vinyl acetate. There is
limited evidence in experimental animals for the carcinogenicity
of vinyl acetate. Therefore, IARC has classified vinyl
acetate as possibly carcinogenic to humans (Group 2B). This
conclusion was based on the following evidence: vinyl
acetate is rapidly transformed into acetaldehyde in human
blood and animal tissues, there is sufficient evidence in
experimental animals for the carcinogenicity of acetaldehyde,
both vinyl acetate and acetaldehyde induce nasal
cancer in rats after administration by inhalation, and vinyl
acetate and acetaldehyde are genotoxic in human cells in
vitro and in animals in vivo .
輸送方法
UN1301 Vinyl acetate, stabilized, Hazard Class: 3;
Labels: 3-Flammable liquid.
純化方法
Inhibitors such as hydroquinone and other impurities are removed by drying with CaCl2 and fractionally distilling under nitrogen, then refluxing briefly with a small amount of benzoyl peroxide and redistilling it under nitrogen. Store it in the dark at 0o. Add inhibitor (~0.004%) for storage. [Beilstein 2 IV 176.]
不和合性
Vinyl acetate may undergo spontaneous
exothermic polymerization on exposure to light. Vapors
may form explosive mixture with air. Incompatible with oxidizers
(chlorates, nitrates, peroxides, permanganates, perchlorates,
chlorine, bromine, fluorine, etc.); contact may
cause fires or explosions. Keep away from alkaline materials,
strong bases, strong acids, oxoacids, epoxides, strong
light and UV. The vapor may react vigorously with silica
gel or aluminum, acids, bases, silica gel; alumina, oxidizers,
azo compounds. Ozone readily polymerizes in elevated temperatures,
under the influence of light, or peroxides. Usually
contains a stabilizer to prevent polymerization.
廃棄物の処理
Dissolve or mix the material
with a combustible solvent and burn in a chemical
incinerator equipped with an afterburner and scrubber. All
federal, state, and local environmental regulations must be
observed.
酢酸ビニル (モノマー) 上流と下流の製品情報
原材料
準備製品