アクリル酸 化学特性,用途語,生産方法
外観
白色/無色粉末~塊~透明液体
定義
本品は、次の化学式で表される有機化合物である。
溶解性
水, アルコール, エーテルに混和。水、エタノール及びアセトンに極めて溶けやすい。
解説
propenoic acid.C3H4O2(72.06).CH2=CHCOOH.実験室的には,アクリル酸エステルのけん化,アクリルアルデヒドの酸化,ヒドロキシプロピオン酸の脱水,およびハロプロピオン酸の脱ハロゲン化水素などの方法があるが,工業的には,次式に示すような合成法がある.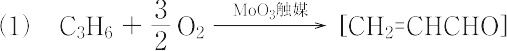 " → CH2=CHCOOH + H2O(プロペンの直接酸化法)
" → CH2=CHCOOH + H2O(プロペンの直接酸化法) 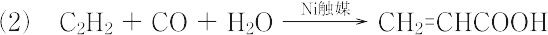 "(レッペ法) 不快な刺激臭をもつ液体.融点13 ℃,沸点141 ℃.d201.0511.n20D1.4224.アクリル酸は,水に可溶.非常に重合しやすいので重合防止剤としてヒドロキノンを加えて貯蔵する.エテンやアクリロニトリルとの共重合体は酸性高分子電解質として重要であり,また水溶液性高分子の原料,そのほか種々の有機合成の原料としても用いられる.アルキルエステルの重合体は,塗料,接着剤などに広く利用される.最近では,ポリアクリル酸を架橋したものが高吸水性樹脂として,おむつ,衛生用品,農業,電子産業などのさまざまな方面で利用されている.皮膚,粘膜,眼を刺激する.LD50 60 mg/kg(マウス,経口).森北出版「化学辞典(第2版)
"(レッペ法) 不快な刺激臭をもつ液体.融点13 ℃,沸点141 ℃.d201.0511.n20D1.4224.アクリル酸は,水に可溶.非常に重合しやすいので重合防止剤としてヒドロキノンを加えて貯蔵する.エテンやアクリロニトリルとの共重合体は酸性高分子電解質として重要であり,また水溶液性高分子の原料,そのほか種々の有機合成の原料としても用いられる.アルキルエステルの重合体は,塗料,接着剤などに広く利用される.最近では,ポリアクリル酸を架橋したものが高吸水性樹脂として,おむつ,衛生用品,農業,電子産業などのさまざまな方面で利用されている.皮膚,粘膜,眼を刺激する.LD50 60 mg/kg(マウス,経口).森北出版「化学辞典(第2版)
用途
アクリル酸(GAA)はプロピレンを原料として直接酸化して製造されます。
各種エステルの原料、高吸水性樹脂(SAP)、分散剤、凝集剤、増粘剤、粘接着剤等の原料として使用されています。
用途
アクリル酸エステル、アクリロニトリル、ブタジエン、酢酸ビニルなどほかのモノマーと共重合させたものは、不織布バインダー、フロッキー加工用バインダー、繊維の改質剤などとして使用される。またポリアクリル酸塩類は高吸水性樹脂、増粘剤、凝集剤の用途がある
化粧品の成分用途
人工爪剤
主な用途/役割
アクリル樹脂原料、反応性アクリル樹脂系接着剤に使用される。
説明
Acrylic acid (IUPAC: prop-2-enoic acid) is an organic compound with the formula CH
2=CHCO
2H. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a characteristic acrid or tart smell. It is miscible with water, alcohols, ethers, and chloroform. More than one billion kilograms are produced annually.
化学的特性
Acrylic acid is a colorless, flammable, and corrosive liquid or solid (below 13 C) with an irritating, rancid, odor. Sinks and mixes with water; irritating vapor is produced.
使用
Acrylic acid is produced by oxidation of acrolein or hydrolysis of acrylonitrile. It is used in the manufacture of plastics; in paints, polishes, and adhesives; and as coatings for leather.
定義
An unsaturated liquid carboxylic acid with a pungent odor.
The acid and its esters are used to make
ACRYLIC RESINS.
調製方法
Acrylic acid is produced from propene which is a by product of ethylene and gasoline production. CH
2=CHCH
3 + 1.5 O
2→ CH
2=CHCO
2H + H
2O Because acrylic acid and its esters have long been valued commercially, many other methods have been developed but most have been abandoned for economic or environmental reasons. An early method was the hydrocarboxylation of acetylene ("Reppe chemistry") : HCCH + CO + H
2O → CH
2=CHCO
2H This method requires nickel carbonyl and high pressures of carbon monoxide. It was once manufactured by the hydrolysis of acrylonitrile which is derived from propene by ammoxidation, but was abandoned because the method cogenerates ammonium derivatives. Other now abandoned precursors to acrylic acid include ethenone and ethylene cyanohydrin.
一般的な説明
Acrylic acid is a colorless liquid with a distinctive acrid odor. Flash point 130°F. Boiling point 286°F. Freezing point 53°F. Corrosive to metals and tissue. Prolonged exposure to fire or heat can cause polymerization. If polymerization takes place in a closed container, violent rupture may occur. The inhibitor (usually hydroquinone) greatly reduces the tendency to polymerize.
空気と水の反応
Flammable. Soluble in water. The presence of water, due to different solubilities of the acid and inhibitor (partitioning one from the other), may initiate polymerization.
反応プロフィール
ACRYLIC ACID may polymerize violently especially when the frozen acid is partially thawed (freezing point 12°C or 53°F). Frozen acid should be melted at room temperature and the process should be well stirred. Do not use heat during the melting process [Kirk-Othmer, 3rd ed., Vol. 1, 1978, p. 330]. Corrodes iron and steel and polymerization may occur on contact with iron salts. The uninhibited acid polymerizes exothermically at ambient temperature and explodes if confined. The inhibitor (usually hydroquinone) greatly reduces the tendency to polymerize. Explosive polymerization can also occur with strong bases, amines, ammonia, oleum, chlorosulfonic acid, and peroxides. Mixing with 2-aminoethanol, 28% ammonium hydroxide, ethylenediamine or ethyleneimine in a closed container causes an increase in temperature and pressure. Can react violently with oxidizing reagents and strong bases [Bretherick, 5th ed., 1995, p. 419].
健康ハザード
Acrylic acid is a corrosive liquid that cancause skin burns. Spill into the eyes candamage vision. The vapors are an irritantto the eyes. The inhalation hazard is oflow order. An exposure to 4000 ppm for4 hours was lethal to rats. The oral LD50values reported in the literature show widevariation. The dermal LD50 value in rabbitsis 280 mg/kg.
火災危険
Combustible liquid; flash point (closed cup)
54°C (130°F), (open cup) 68°C (155°F);
vapor pressure 31 torr at 25°C (77°F); vapor
density 2.5 (air=1); autoignition temperature 360°C (680°F). Vapors of acrylic acid
form explosive mixtures with air within the
range 2.9–8.0% by volume in air. Fireextinguishing agent: water spray, “alcohol”
foam, dry chemical, or CO2; use a water
spray to flush and dilute the spill and to disperse the vapors.
Acrylic acid may readily polymerize at
ambient temperature. Polymerization may
be inhibited with 200 ppm of hydroquinone
monomethyl ether (Aldrich 2006). In the
presence of a catalyst or at an elevated temperature, the polymerization rate may accelerate, causing an explosion. The reactions of
acrylic acid with amines, imines, and oleum
are exothermic but not violent. Acrylic acid
should be stored below its melting point with
a trace quantity of polymerization inhibitor.
Its reactions with strong oxidizing substances
can be violent.
使用用途
アクリル酸は主にプロピレンの直接酸化によって製造されています。直接酸化法とはプロピレンを酸化させることでアルデヒドであるに変換し、さらに酸化させることでアクリル酸を製造する方法です。
アクリル酸は紙おむつなどに使われる高吸水性樹脂(SAP)であるポリアクリル酸の原料として用いられています。その他、アクリル酸をエステル化した、などのアクリル酸エステルはアクリル繊維の原料などに用いられています。その他にもアクリル酸、アクリル酸エステルは、合成樹脂、分散剤や凝集剤、増粘剤などの原料として使われています。
安全性と法規制
アクリル酸の安全性と法規制
アクリル酸は常温で液体、特徴的な刺激臭を有する物質で、消防法第4類の第二石油類に該当する引火性の物質です。また、アクリル酸は分子内に炭素-炭素二重結合を有しており、重合反応が起こる可能性があります。一般的には酸素濃度の管理や重合禁止剤を添加して重合を抑制していますが、加熱や光などで反応が起こり、反応熱によって反応が促進、暴走する可能性があります。
その他、アクリル酸は毒物及び劇物取締法の劇物に該当する物質であるほか、皮膚腐食性、刺激性がある物質でもあります。また、アクリル酸はPRTR法の第1種指定化学物質であり、労働安全衛生法上のリスクアセスメント対象物質でもあり、適切な管理、評価が求められる物質です。
接触アレルゲン
Acrylates are esters from acrylic acid. Occupational
contact allergies from acrylates have frequently been
reported and mainly concern workers exposed to the
glues based on acrylic acid, as well as dental workers
and beauticians.
安全性プロファイル
Poison by ingestion, skin contact, and intraperitoneal routes. An experimental teratogen. Other experimental reproductive effects. A severe skin and eye irritant. Questionable carcinogen with experimental carcinogenic and tumorigenic data. Corrosive. Flammable liquid. May undergo exothermic polymerization at room temperature. May become explosive if confined. A fire hazard when exposed to heat or flame.
安全性
Acrylic acid is severely irritating and corrosive to the skin and the respiratory tract. Eye contact can result in severe and irreversible injury. Low exposure will cause minimal or no health effects, while high exposure could result in pulmonary edema. The LD
50 is 340 mg/kg (rat, oral).
職業ばく露
Acrylic acid is chiefly used in manufacture of plastics, acrylates, polyacrylic acids, polymer, and resins; as a monomer in the manufacture of acrylic resins and plastic products, leather treatment, and paper coatings. Also, it is used as a tackifier and flocculant.
環境運命予測
Acrylic acid is corrosive, and its toxicity occurs at the site of
contact.
輸送方法
UN2218 Acrylic acid, stabilized, Hazard class: 8; Labels: 8-Corrosive material, 3-Flammable liquid
純化方法
It can be purified by steam distillation, or vacuum distillation through a column packed with copper gauze to inhibit polymerisation. (This treatment also removes inhibitors such as methylene blue that may be present.) Azeotropic distillation of the water with *benzene converts aqueous acrylic acid to the anhydrous material. [Beilstein 2 H 397, 2 I 186, 2 II 383, 2 III 1215, 2 IV 1455.]
不和合性
May form explosive mixture with air. Light, heat, and peroxides can cause polymerization. Use MEHQ (monomethyl ether of hydroquinone) as an inhibitor. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Incompatible with sulfuric acid, caustics, ammonia, amines, isocyanates, alkylene oxides; epichlorohydrin, toluene diamine, oleum, pyridine, methyl pyridine, n-methyl pyrrolidone, 2-methyl-6-ethyl aniline, aniline, ethylene diamine, ethyleneimine, and 2aminoethanol. Severely corrodes carbon steel and iron; attacks other metals. May accumulate static electrical charges and may cause ignition of its vapors.
廃棄物の処理
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration. 100 500 ppm potassium permanganate will degrade acrylic acid to a hydroxy acid which can be disposed of at a sewage treatment.
アクリル酸 上流と下流の製品情報
原材料
準備製品
Carboxy styrene-butadiene latex
water stabilizing agent YSS-93
3-Hydrazinyl-N-methylpropanamide
Acrylic latex
Water quality stabilizer
AMPS-Acrylate copolymer
アクリル酸 2- エチルヘキシル,モノマー
2-ホスホノブタン-1,2,4-トリカルボン酸
アクリル酸ヒドロキシプロピル (2-ヒドロキシプロピルエステル, 2-ヒドロキシ-1-メチルエチルエステル混合物)
4-クロロ-8-(トリフルオロメチル)キノリン
1,2,3,4-テトラヒドロ-9-メチルカルバゾール-4-オン
アクリル酸4-ヒドロキシブチル
Pressure sensitive adhesive
Latex paint for interior and exterior wall
nonformaldehyde resin finishing agent CN-NF^{3^}
N-(アクリロイルオキシ)スクシンイミド
2-(2-CARBOXYETHYL)THIO-4,6-DIMETHYLPYRIMIDINE
アクリル酸重合物のナトリウム塩
アクリル酸-2-ヒドロキシプロピル アクリル酸-メチル アクリル酸共重合物
Antiscale and inhibitor
acrylic resin coating finish
Viscosity increaser
Antiscale dispersant
ぞうちょうざい
PAINT
トリアクリル酸2-ヒドロキシエタン-1,1,1-トリイルトリスメチレン
Ce^<4+^> initiated starch-g-acrylic acid water absorbent agent (I)
scale inhibitor and dispersant TS-1615
conductive coating-composite system of acrylic copolymer and cuprous iodide
ポリ(アクリル酸-CO-マレイン酸) 溶液
3-(1H-1,2,4-トリアゾール-1-イル)プロパン酸
トリメチロ-ルプロパンEO付加トリアクリレ-ト
Styrene-acrylic latex
Water treatment disinfectant
Binder for coating printing
corrosion and scale inhibitor CW-1901
surfactant ASMS
アクリル酸エチル
acrylate resin emulsion s-1
ポリアクリル酸ナトリウム