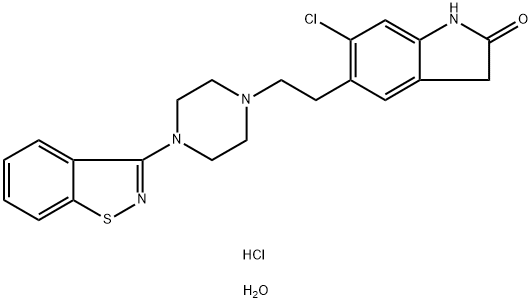
Ziprasidone hydrochloride monohydrate
|
|
Ziprasidone hydrochloride monohydrate 속성
- 녹는점
- 300°C
- 저장 조건
- 2-8°C
- 용해도
- DMSO: >10mg/mL
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 색상
- 밝은 오렌지색
- Merck
- 14,10171
- BCS Class
- 2?
- CAS 데이터베이스
- 138982-67-9(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 안전지침서 | 24/25 | ||
|---|---|---|---|
| 유엔번호(UN No.) | UN1230 - class 3 - PG 2 - Methanol, solution | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | NM3241200 | ||
| HS 번호 | 38220010 |
| 그림문자(GHS): |
 
|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 신호 어: | Warning | |||||||||||||||||||||
| 유해·위험 문구: |
|
|||||||||||||||||||||
| 예방조치문구: |
|
Ziprasidone hydrochloride monohydrate C화학적 특성, 용도, 생산
화학적 성질
Pale Tan Powder용도
Combined serotonin (5HT2) and dopamine (D2) receptor antagonist. Used as an antipsychotic.정의
ChEBI: The hydrochloride hydrate salt of ziprasidone.생물학적 활성
Atypical antipsychotic that displays combined serotonin and dopamine receptor antagonism. Displays high affinity at 5-HT 2A receptors with a 5-HT 2A /D 2 affinity ratio greater than any other clinically available atypical antipsychotics (pK i values are 9.38, 8.88, 8.69, 8.47, 8.32, 8.14, 7.98, 7.49, 7.33 and 6.28 for 5-HT 2A , 5-HT 2C , 5-HT 1D , 5-HT 1A , D 2 , D 3 , α 1 , D 4 , H 1 and D 1 receptors respectively).Ziprasidone hydrochloride monohydrate 준비 용품 및 원자재
원자재
Serotonin hydrochloride
Benzoisothiazole
피페라진
인돌
이소프로필알코올
p-터페닐
3-(1-Piperazinyl)-1,2-benzisothiazole
6-Chloro-5-(2-chloroethyl)oxindole
준비 용품
Ziprasidone hydrochloride monohydrate 공급 업체
글로벌( 335)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Xiamen Wonderful Bio Technology Co., Ltd. | +8613043004613 |
Sara@xmwonderfulbio.com | China | 305 | 58 |
| Nantong Guangyuan Chemicl Co,Ltd | +undefined17712220823 |
admin@guyunchem.com | China | 616 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9348 | 55 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 |
sales@alchempharmtech.com | United States | 63711 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49390 | 58 |
Ziprasidone hydrochloride monohydrate 관련 검색:
Ziprasidone
3-(1-Piperazinyl)-1,2-benzisothiazole
Ziprasidone HCl
Zirconium hydroxide
3-Oxo Ziprasidone
(Ziprasidone Impurity B)
1,1-DIMETHYLETHYL 4-(1,2-BENZISOTHIAZOLE-3-YL)-1-PIPERAZINECARBOXYLATE
3-[4-[2-(2,5-Dichloro-4-nitrophenyl)ethyl]-1-piperazinyl]-1,2-benzisothiazole
Hydroxy Ziprasidone
Ziprasidone N-Oxide
5-[2-[4-(1,2-Benzisothiazol-3-yl)-1-piperazinyl]ethyl-4-chloro-2-nitrophenol
6-chloro-5-[2-[4-(2-methylsulfanylbenzenecarboximidoyl)piperazin-1-yl]ethyl]-1,3-dihydroindol-2-one
5,5'-Bis(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)ethyl)-6,6'-dichloro-3-hydroxy-3,3'-biindoline-2,2'-dione
1,2-Benzisothiazole, 3-(1-piperazinyl)-, 1-oxide
3-(1,2-Benzisothiazolyl) Ziprasidone
(Ziprasidone Impurity E)
Ziprasidone Amino Acid
(Ziprasidone Impurity C)
3-[4-[2-(2,5-Dichloro-4-nitrophenyl)ethenyl]-1-piperazinyl]-1,2-benzisothiazole
5-[2-[4-(1,2-Benzisothiazol-3-yl)-1-piperazinyl]ethyl]-4-chloro-2-nitro-benzeneacetic Acid
1,2-Benzisothiazole, 3-(1-piperazinyl)-, 1,1-dioxide