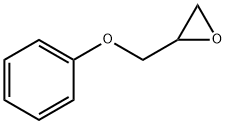
페닐글리시딜에테르
|
|
페닐글리시딜에테르 속성
- 녹는점
- 3.5 °C(lit.)
- 끓는 점
- 245-247 °C(lit.)
- 밀도
- 1.109 g/mL at 25 °C(lit.)
- 증기 밀도
- 5.2 (vs air)
- 증기압
- 0.03 mm Hg ( 20 °C)
- 굴절률
- n
20/D 1.531
- 인화점
- >230 °F
- 저장 조건
- Store below +30°C.
- 용해도
- 2.4g/L
- 물리적 상태
- 액체
- 색상
- 무색~황색으로 투명함
- 수용성
- 2.4g/L(20℃)
- BRN
- 2744
- 노출 한도
- TLV-TWA 6 mg/m3 (1 ppm) (ACGIH); 1 ppm (15 min) (NIOSH).
- 안정성
- 안정적인. 강한 산화제와 호환되지 않습니다.
- InChIKey
- FQYUMYWMJTYZTK-UHFFFAOYSA-N
- LogP
- 1.61 at 25℃
- CAS 데이터베이스
- 122-60-1(CAS DataBase Reference)
- IARC
- 2B (Vol. 47, 71) 1999
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 45-20-37/38-43-52/53-68 | ||
| 안전지침서 | 53-45-61 | ||
| 유엔번호(UN No.) | 2810 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | TZ3675000 | ||
| 자연 발화 온도 | 430 °C | ||
| TSCA | Yes | ||
| 위험 등급 | IRRITANT | ||
| HS 번호 | 29109000 | ||
| 유해 물질 데이터 | 122-60-1(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: 2150 mg/kg LD50 dermal Rabbit 1500 mg/kg | ||
| IDLA | 100 ppm | ||
| 기존화학 물질 | KE-28260 |
페닐글리시딜에테르 C화학적 특성, 용도, 생산
화학적 성질
Phenyl glycidyl ether, also known as glycidyl phenyl ether or PGE, is a clear liquid with a sweet odor that is considered unpleasant. It is soluble in ether and benzene, but insoluble in water. It has the ability to volatilize with water vapor. It is used in the production of epoxy resins, as a chemical intermediate, and as a stabilizer.용도
Phenyl glycidyl ether (PGE) is utilized in organic syntheses as an intermediate. As a reactive diluent, PGE is incorporated into uncured epoxy resins to lower viscosity, thereby facilitating casting, adhesives, and laminating applications.생산 방법
PGE is synthesized by condensation of phenol with epichlorohydrin, with subsequent dehydrochlorination with caustic to form the epoxy ring.일반 설명
Colorless liquid.공기와 물의 반응
Ethers tend to form unstable peroxides when exposed to oxygen. Ethyl, isobutyl, ethyl tert-butyl, and ethyl tert-pentyl ether are particularly hazardous in this respect. Ether peroxides can sometimes be observed as clear crystals deposited on containers or along the surface of the liquid. Slightly soluble in water.반응 프로필
Glycidyl phenyl ether, an ether, can act as a base. They form salts with strong acids and addition complexes with Lewis acids. The complex between diethyl ether and boron trifluoride is an example. Ethers may react violently with strong oxidizing agents. In other reactions, which typically involve the breaking of the carbon-oxygen bond, ethers are relatively inert.건강위험
PGE is a toxic compound exhibiting moderate irritant action and carcinogenicity inanimals. Application of 0.25 mg resulted insevere eye irritation in rabbits, while 500 mgcaused moderate skin irritation over a periodof 24 hours. Prolonged or repeated contactcan cause moderate irritation and skin sensitization in humans.The symptoms of its toxicity in animalswere depression of the central nervous system and paralysis of the respiratory tract.Prolonged exposure caused changes in thekidney, liver, thymus, and testes, and lossof hair in rats. The toxicity of this compound in humans is low and the health hazardcan arise primarily from its skin-sensitizationaction.
LD50 value, oral (mice): 1400 mg/kg
DGE showed carcinogenicity in rats, causingnasal cancer.
화재위험
Glycidyl phenyl ether is probably combustible.색상 색인 번호
This monoglycidyl derivative is a reactive diluent in epoxy resins Bisphenol A type. It is a component of epoxy paints, epoxy glues, and epoxy resins. Sensitization has been observed in many professions, such as in construction workers, marble workers, ceramic workers, and shoemakers.Safety Profile
Confirmed carcinogen with experimental carcinogenic data. Moderately toxic by ingestion, skin contact, and subcutaneous routes. A severe eye and skin irritant. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes. Used as a chemical intermediate. See also ETHERS잠재적 노출
PGE is used to increase storage time and stability of halogenated compounds; as a reactive diluent in uncured epoxy resins to reduce the viscosity of the uncured system for ease in casting; adhesive, and laminating applications. NIOSH once estimated that 8000 workers are potentially exposed to PGE.Carcinogenicity
Chronic exposure of rats to 1 or 12ppm 6 hours/day, 5 days/week for 2 years caused an increased incidence of rhinitis, squamous metaplasia, and epidermal carcinomas of the nasal cavity.4 The IARC has determined that there is sufficient evidence for the carcinogenicity of PGE in animals and that it is possibly carcinogenic to humans.운송 방법
UN2810 Toxic liquids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.비 호환성
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, amines, and curing agents. PGE can presumably form explosive peroxides폐기물 처리
Concentrated waste containing no peroxides-discharge liquid at a controlled rate near a pilot flame. Concentrated waste containing peroxidesperforation of a container of the waste from a safe distance followed by open burning.페닐글리시딜에테르 준비 용품 및 원자재
원자재
준비 용품
페닐글리시딜에테르 공급 업체
글로벌( 242)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5993 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 |
ivan@atkchemical.com | China | 32480 | 60 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 |
sales@alchempharmtech.com | United States | 63711 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49390 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; |
factory@coreychem.com | China | 29826 | 58 |
| BEYOND INDUSTRIES (CHINA) LIMITED | +86-21-52699951; +8613917686115 |
sales@beyondindustriesgroup.com | China | 699 | 58 |