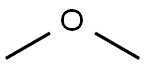
디메틸 에테르
|
|
디메틸 에테르 속성
- 녹는점
- −141 °C(lit.)
- 끓는 점
- −24.8 °C(lit.)
- 밀도
- 1.617
- 증기 밀도
- 1.62 (vs air)
- 증기압
- >760 mm Hg ( 25 °C)
- 굴절률
- 1.2984
- 인화점
- -41°C
- 용해도
- Soluble in acetone, chloroform, ethanol (95%), ether, and 1 in 3 parts of water. Dimethyl ether is generally miscible with water, nonpolar materials, and some semipolar materials. For pharmaceutical aerosols, ethanol (95%) is the most useful cosolvent. Glycols, oils, and other similar materials exhibit varying degrees of miscibility with dimethyl ether.
- 냄새
- 클로로포름 유사; 달콤한.
- 폭발한계
- 27%
- Odor Threshold
- 500ppm
- 수용성
- 녹는
- Merck
- 6071
- BRN
- 1730743
- Dielectric constant
- 5.0(25℃)
- 안정성
- 안정적인. 가연성이 매우 높습니다. 낮은 인화점을 참고하세요. 공기와 폭발성 혼합물을 형성할 수 있음. 장기간 보관하는 동안 과산화물이 형성될 수 있습니다.
- LogP
- 0.07 at 25℃
- CAS 데이터베이스
- 115-10-6(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | F+ | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 12 | ||
| 안전지침서 | 9-16-33 | ||
| 유엔번호(UN No.) | UN 1033 2.1 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | PM4780000 | ||
| F 고인화성물질 | 4.5-31 | ||
| 자연 발화 온도 | 662 °F | ||
| 위험 등급 | 2.1 | ||
| HS 번호 | 2909199090 | ||
| 유해 물질 데이터 | 115-10-6(Hazardous Substances Data) | ||
| 기존화학 물질 | KE-27704 |
디메틸 에테르 C화학적 특성, 용도, 생산
화학적 성질
Dimethyl ether is a liquefied gas and exists as a liquid at room temperature when contained under its own vapor pressure, or as a gas when exposed to room temperature and pressure.It is a clear, colorless, virtually odorless liquid. In high concentrations, the gas has a faint ether-like odor.
용도
Dimethyl ether is used as a solvent in aerosol formulations.생산 방법
Dimethyl ether is prepared by the reaction of bituminous or lignite coals with steam in the presence of a finely divided nickel catalyst. This reaction produces formaldehyde, which is then reduced to methanol and dimethyl ether. Dimethyl ether may also be prepared by the dehydration of methanol.정의
ChEBI: An ether in which the oxygen atom is connected to two methyl groups.일반 설명
Dimethyl ether is a colorless gas with a faint ethereal odor. Dimethyl ether is shipped as a liquefied gas under its vapor pressure. Contact with the liquid can cause frostbite. Dimethyl ether is easily ignited. Its vapors are heavier than air. Any leak can be either liquid or vapor. Dimethyl ether can asphyxiate by the displacement of air. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket.공기와 물의 반응
Highly flammable. Upon standing and exposure to air (oxygen) tendency to form explosive peroxides. When ethers containing peroxides are heated (distilled) they can detonate [Lewis, 3rd ed., 1993, p. 854].반응 프로필
Dimethyl ether is a colorless, highly flammable gas (b. p. -24° C), slightly toxic. Very dangerous fire and explosion hazard when exposed to flame, sparks, heat or strong oxidizers. Violent reaction with aluminum hydride, lithium aluminum hydride. Upon standing and exposure to air (oxygen) tendency to form explosive peroxides. When ethers containing peroxides are heated (distilled) they can detonate [Lewis, 3rd ed., 1993, p. 854].건강위험
Methyl ether produced low inhalation toxicity in rats. Caprino and Togna (1975)reported a 30-minute LC50 value of 396 ppmfor rats. In lethal doses it caused sedation,a gradual depression of motor activity, lossof the sighting reflex, hypopnea, coma, anddeath in mice. Exposure to a 40% mixtureof methyl ether in air resulted in an initialslight increase in heart rate in rabbits, whichwas followed by depression of arterial bloodpressure. Death occurred in 45 minutes. Thearterial and venous partial oxygen pressurewas found to decrease while the venous CO2pressure and the blood pH increasedReuzel et al. (1981) reported that sub chronic inhalation of methyl ether in ratsdid not cause significant adverse effects.No noticeable effect on organ and bodyweights and no treatment-related changeswere observed. In humans, adverse healtheffect from inhalation of this compoundshould be minimal. However, inhalation ofexcessive quantities can produce intoxicationand loss of consciousness.
화재위험
Behavior in Fire: Containers may explode. Vapors are heavier than air and may travel long distance to a source of ignition and flash back.화학 반응
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.Pharmaceutical Applications
Dimethyl ether may be used as an aerosol propellant for topical aerosol formulations in combination with hydrocarbons and other propellants. Generally, it cannot be used alone as a propellant owing to its high vapor pressure. Dimethyl ether is a good solvent and has the unique property of high water solubility, compared to other propellants. It has frequently been used with aqueous aerosols. A coarse, wet, spray is formed when dimethyl ether is used as a propellant.Dimethyl ether is also used as a propellant in cosmetics such as hair sprays,and in other aerosol products such as air fresheners and fly sprays.
Dimethyl ether is additionally used as a refrigerant.
Safety
Dimethyl ether may be used as a propellant and solvent in topical pharmaceutical aerosols, and is generally regarded as an essentially nontoxic and nonirritant material when used in such applications. However,inhalation of high concentrations of dimethyl ether vapor is harmful. Additionally, skin contact with dimethyl ether liquid may result in freezing of the skin and severe frostbite.When used in topical formulations, dimethyl ether may exert a chilling effect on the skin, although if it is used as directed the propellant quickly vaporizes and is nonirritating.
LD50 (mouse, inhalation): 386000ppm/30min
LD50 (rat, inhalation): 308g/m3
Carcinogenicity
A lifetime study in rats did not produce cancer or clear, statistically significant evidence of chronic toxicity at 25,000 ppm of dimethyl ether.환경귀착
DME released to the atmosphere would be expected to exist almost entirely in the vapor phase since the vapor pressure is 4450 mmHg at 25 ℃. It is susceptible to photooxidation via vapor phase reaction with photochemically produced hydroxyl radicals. An atmospheric half-life of 5.4 days has been calculated. It will also exhibit very highmobility in soil and, therefore, itmay leach to groundwater. If DME is released to water, it will not be expected either to significantly absorb to sediment or suspended particulate matter, bioconcentrate in aquatic organisms, or directly photolyze. No data concerning the biodegradation of DME in environmental media were located but many ethers are known to be resistant to biodegradation. DME would not be expected to bioconcentrate in aquatic organisms.저장
The liquefied gas is stable when used as a propellant. However, exposure to the air for long periods of time may result in explosive peroxides being slowly formed.Solutions of liquid dimethyl ether should not be concentrated either by distillation or by evaporation. Dimethyl ether should be stored in tightly closed metal cylinders in a cool, dry place.
Purification Methods
Dry methyl ether by passing over alumina and then BaO, or over CaH2, followed by fractional distillation at low temperatures. Its solubility is 37mL per mL of H2O at 18o, and it is very soluble비 호환성
Dimethyl ether is an aggressive solvent and may affect the gasket materials used in aerosol packaging. Oxidizing agents, acetic acid, organic acids, and anhydrides should not be used with dimethyl ether.Regulatory Status
Included in the FDA Inactive Ingredients Database (topical aerosols). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.디메틸 에테르 준비 용품 및 원자재
원자재
준비 용품
3- 아미노 디 페닐
아네톨
3-아미노-4-메톡시아세트아닐라이드
2,5-Diaminoanisole sulfate
p-브로모아니졸
N-메틸아닐린
N-(4-METHOXY-3-NITROPHENYL)ACETAMIDE
2-플루오로-4-하이드록시벤조니트릴
아니졸
4-아이오딘아니솔
3-Methoxy-18-methylestra-2,5(10)dien-17-one 17-ethylene ketal
플루싸이트린에이트
3-PYRID-2-YLBENZOIC ACID
5-METHYLFURAN-2-BORONIC ACID
1,2-다이메톡시에테인
클로로메틸 메틸 에테르
테트라하이드라피란
디메틸 에테르 공급 업체
글로벌( 88)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49390 | 58 |
| SIMAGCHEM CORP | +86-13806087780 |
sale@simagchem.com | China | 17367 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 |
sales@tnjchem.com | China | 34572 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 |
1026@dideu.com | China | 29220 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 |
1026@dideu.com | China | 9116 | 58 |
| Shanghai Quedan biology science and technology co., ltd | +86-13004146052 |
lnzc@quedan.com.cn | China | 1060 | 58 |
| PT CHEM GROUP LIMITED | +86-85511178 +86-85511178 |
peter68@ptchemgroup.com | China | 35453 | 58 |
| ZHEJIANG JIUZHOU CHEM CO., LTD | +86-0576225566889 +86-13454675544 |
admin@jiuzhou-chem.com;jamie@jiuzhou-chem.com;alice@jiuzhou-chem.com | China | 20000 | 58 |