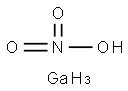갈륨 니트레이트
|
|
갈륨 니트레이트 속성
- 녹는점
- 110°C (dec.)
- 용해도
- soluble in H2O, ethanol, ethyl ether
- 물리적 상태
- 액체
- 색상
- white crystals, crystalline powder
- 수용성
- 물에 용해됩니다.
- 노출 한도
- ACGIH: TWA 2 ppm; STEL 4 ppm
OSHA: TWA 2 ppm(5 mg/m3)
NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험 카페고리 넘버 | 8-36/37/38 | ||
|---|---|---|---|
| 안전지침서 | 17-26-36/37/39 | ||
| 유엔번호(UN No.) | 1477 | ||
| TSCA | Yes | ||
| 위험 등급 | 5.1 | ||
| 포장분류 | III | ||
| 기존화학 물질 | KE-17446 |
갈륨 니트레이트 C화학적 특성, 용도, 생산
개요
Gallium nitrate, initially developed as an anticancer agent, was introduced by Fujisawa as an orphan drug for the treatment of cancer-related hypercalcemia and bone metastases that do not respond to adequate hydration. The compound acts specifically on bone by inhibiting calcium resorption and also possibly by stimulating bone formation. Compared with calcitonin and etidronate, gallium nitrate is more potent and substantially longer acting. Other potential uses could be in the treatment of osteoporosis and Paget’s disease.화학적 성질
The structural formula of gallium nitrate is Ga(NO3)3. Gallium nitrate is an anhydrate salt that is very soluble in water and soluble in 95% ethanol. It is stable in commonly used intravenous fluids for 14 days at room temperature and at 5 ℃, and physically compatible for injection with selected drug products, with the exception of diazepam injection.This chemical is an oxidizer, and is probably combustible용도
Gallium is the second metal ion with clinical activity used in cancer treatment. Gallium nitrate has demonstrated antitumor activity in a variety of murine tumor models, including Walker 256 carcinosarcoma, fibrosarcoma M-89, leukemia K-1964, adenocarcinoma 755, mammary carcinoma YMC, reticulum cell sarcoma A-RCS, lymphoma P1798, and osteosarcoma 124F. It was, however, ineffective in ascites, leukemias, plasma cell tumors, or Ehrlich carcinoma. In phase II evaluation studies, gallium nitrate has shown antitumor activity in patients with either refractory lymphomas or small-cell lung carcinomas or bladder cancer, with total objective response rates of 28% and 11%, respectively. This drug has displayed its strongest antineoplastic activity in the treatment of non-Hodgkin’s lymphoma and bladder cancer.In addition, according to two phase III comparative trials,thanks to an inhibitory effect on calcium reabsorption from bone, gallium nitrate is superior to alternative therapies in the treatment of hypercalcemia associated with malignancy and related disease states. Based on its clinical efficacy, gallium nitrate (Ganite ) was approved by the Food and Drug Administration (FDA) for the treatment of cancerassociated hypercalcemia.
Apart from its ability to control hypercalcemia, gallium nitrate has been shown to inhibit bone turnover and decrease osteolysis in patients with multiple myeloma and in patients with bone metastases from a variety of different cancers.
일반 설명
White crystals.공기와 물의 반응
Deliquescent. Water soluble.반응 프로필
Oxidizing agents, such as GALLIUM NITRATE, can react with reducing agents to generate heat and products that may be gaseous (causing pressurization of closed containers). The products may themselves be capable of further reactions (such as combustion in the air). The chemical reduction of materials in this group can be rapid or even explosive, but often requires initiation (heat, spark, catalyst, addition of a solvent). Explosive mixtures of inorganic oxidizing agents with reducing agents often persist unchanged for long periods if initiation is prevented. Such systems are typically mixtures of solids, but may involve any combination of physical states. Some inorganic oxidizing agents are salts of metals that are soluble in water; dissolution dilutes but does not nullify the oxidizing power of such materials. Organic compounds, in general, have some reducing power and can in principle react with compounds in this class. Actual reactivity varies greatly with the identity of the organic compound. Inorganic oxidizing agents can react violently with active metals, cyanides, esters, and thiocyanates.화재위험
Flash point data for GALLIUM NITRATE are not available, but GALLIUM NITRATE is probably combustible.Pharmaceutical Applications
In clinical trials, gallium nitrate has proved to be highly active as an antitumour agent especially against non-Hodgkin’s lymphoma and bladder cancer. The cytotoxic activity of gallium nitrate has been demonstrated as single agent and as part of combination therapy, for example, together with fluorouracil. Gallium nitrate shows a relatively low toxicity and does not produce myelosuppression, which is a significant advantage over other traditional anticancer agents. Furthermore, it does not appear to show any cross-resistance with conventional chemotherapeutic agents.These studies have also shown that gallium nitrate is able to decrease serum calcium levels in patients with tumour-induced hypercalcaemia. Subsequently, several studies have been carried out comparing traditional bisphosphonate drugs with gallium nitrate in their ability to decrease the calcium levels that are elevated as a result of cancer. Based on the clinical efficacy, gallium nitrate injections (GaniteTM) was granted approval by the FDA for the treatment of cancer-associated hypercalcaemia. Gallium nitrate is also believed to inhibit the bone turnover and therefore to decrease osteolysis, the active reabsorption of bone material, in patients with bone metastasis secondary to other cancers.
Carcinogenicity
Studies on the antitumor activity of gallium nitrate have shown that it is particularly active against solid tumors. It has demonstrated antitumor activity in a variety of murine tumor models, including Walker carcinosarcoma 256, fibrosarcoma M-89, leukemia K-1964, adenocarcinoma 755, mammary carcinoma YMC, reticulum cell sarcoma A-RCS, lymphoma P1798, and osteosarcoma 124F.갈륨 니트레이트 준비 용품 및 원자재
원자재
준비 용품
갈륨 니트레이트 공급 업체
글로벌( 66)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5873 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39916 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
factory@coreychem.com | China | 29823 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 |
info@antaichem.com | CHINA | 9641 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 |
sales@tnjchem.com | China | 34571 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 |
1026@dideu.com | China | 8232 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 |
market18@leapchem.com | China | 24738 | 58 |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 |
sales@molcore.com | China | 49739 | 58 |
| Meryer (Shanghai) Chemical Technology Co., Ltd. | 021-61259108 18621169109 |
market03@meryer.com | China | 40240 | 62 |
갈륨 니트레이트 관련 검색:
니켈 나이트레이트 헥사하이드레이트 갈륨 갈륨 니트레이트
Samarium nitrate
Ceric ammonium nitrate
Lanthanum(III) nitrate hexahydrate
LANTHANUM NITRATE HYDRATE
Holmium(III) nitrate pentahydrate
TERBIUM(III) ACETATE HYDRATE
THULIUM(III) ACETATE HYDRATE
Gadolinium nitrate
INDIUM ACETATE
Scandium(III) nitrate
ANTIMONY (IV) OXIDE
RUBIDIUM
Germanium
Molybdenum carbide
Cobaltous nitrate hexahydrate