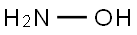히드록실아민 C화학적 특성, 용도, 생산
개요
Hydroxylamine was first synthesized by Wilhem Clemens
Lossen in 1865 in the laboratory of Wilhelm Heinrich Heintz
while working in Halle. The Lossen synthesis originally generated
hydroxylamine in aqueous solution. Anhydrous hydroxylamine
was prepared later by Lobry de Bruyn and Crismer in
1891. The free base is extremely volatile, and industrial-scale
production has been fraught with problems, including large
explosions at facilities in the United States and Japan. Much of
the hydroxylamine produced and transported is in salt form or
as a dilute aqueous solution.
화학적 성질
slightly yellow liquid
물리적 성질
White crystalline solid; orthogonal plates or needles; unstable; density 1.21g/cm3at 20°C; melts at 33°C; vaporizes at 58°C; very soluble in water, liquidammonia and lower alcohols; sparingly soluble in most other organic solvents;decomposes in hot water; pKa5.94 at 25°C.
용도
Hydroxylamine is used as a reducing agent in photography, in
synthetic and analytical chemistry, as an antioxidant for fatty
acids and soaps, and as a dehairing agent for hides. In addition,
hydroxylamine is used in the production of cyclohexanone
oxime, an isomer of caprolactam, which is an intermediate in
the production of nylon-6. In the semiconductor industry,
hydroxylamine can be a component of a solution that dissolves
a photoresist following lithography. Hydroxylamine can also
be used to selectively cleave asparaginyl-glycine peptide bonds.
정의
ChEBI: The simplest hydroxylamine, consisting of ammonia bearing a hydroxy substituent. It is an intermediate in the biological nitrification by microbes like bacteria.
제조 방법
Hydroxylamine is unstable as a free base. It is prepared from hydroxy-lamine hydrochloride, NH2OH?HCl, which is obtained by electrolytic reduc-tion of ammonium chloride solution. The hydrochloride undergoes alkalinedecomposition to hydroxylamine, which is collected by vacuum distillation.
일반 설명
Odorless white crystalline solid. Sinks and mixes with water.
공기와 물의 반응
Decomposes rapidly at room temperature or when dissolved in hot water by internal oxidation-reduction. Reacts with water or steam to produce heat and corrosive liquids.
반응 프로필
HYDROXYLAMINE is a white solid, thermally unstable, decomposes rapidly at room temperature or when dissolved in hot water by internal oxidation-reduction. HYDROXYLAMINE should be stored below 10° C [Bailar, 1973, vol. 2, p. 272]. Explosive reaction with strong oxidizers (chromium trioxide, potassium dichromate) or powdered zinc upon heat. Reaction with zinc or calcium produces explosive bishydroxylamides. HYDROXYLAMINE ignites on contact with cupric sulfate, alkali metals (sodium, potassium), oxidants (e.g., barium oxide, barium peroxide, lead dioxide, potassium permanganate, chlorine), phosphorus trichloride and pentachloride. HYDROXYLAMINE reacts vigorously with hypochlorites, pyridine, carbonyls [Sax, 9th ed., 1996, p. 1875]. On contact with organic materials in thin layer (e.g., crystals on filter paper), HYDROXYLAMINE may ignite spontaneously in air. HYDROXYLAMINE explodes when heated above 70° C [Brauer, 1963, vol. 1, p. 502]. During a distillation process, an explosion occurred. Potassium hydroxide is thought to be involved in the explosion. Employees in the plant complained of chest pains and suffered chemical burns. Five people were killed by the explosion.
위험도
Decomposes rapidly at room temperature,
violently when heated, detonates in flame-heated
test tube. Irritant to tissue.
건강위험
INHALATION: Moderately toxic by inhalation and oral routes with the following symptoms possible: headache, vertigo, tinnitus, dyspnea, nausea and vomiting, cyanosis, proteinuria and hematuria, jaundice, restlessness, and convulsion. Methemoglobinemia has been reported. EYES: Corrosive - highly irritating. SKIN: Irritating or corrosive to skin. INGESTION: Moderately toxic by inhalation and oral routes with the following symptoms possible; headache, vertigo, tinnitus, dyspnea, nausea and vomiting, cyanosis, proteinuria and hematuria, jaundice, restlessness, and convulsion. Methemoglobinemia has been reported.
색상 색인 번호
Hydroxylamine and its salts are used in various
branches of industry, as reducing agents in color film
developers or as reagents in laboratories.
Carcinogenicity
Carcinogenicity of hydroxylamine and
its salts has not been demonstrated. Several
studies have shown a decreased incidence of
spontaneous mammary tumors in mice exposed
to the sulfate and hydrochloride.3–7 There was
some indication of an increase in the incidence
of spontaneous mammary tumors when the
sulfate was administered to older animals
whose mammary glands were already well
developed.
환경귀착
The large-scale production and use of hydroxylamine may
result in its release to the environment through various waste
streams. Hydroxylamine will exist solely as a vapor in the
ambient atmosphere, and will be degraded in the atmosphere
by reaction with photochemically produced hydroxyl radicals;
the half-life for this reaction in air is estimated to be 18 h.
Abiotic degradation of hydroxylamine by photochemically
produced peroxy radicals is an important environmental fate
process in surface waters, with the half-life of the reaction
measured at approximately 2 h. An estimated bioconcentration
factor of 3 suggests that the potential for bioconcentration in
aquatic organisms is low. If released terrestrially, hydroxylamine
will most likely exist in its protonated form due to its
pKa of 5.94; the protonated form is nonvolatile. Koc estimates
of 14 for hydroxylamine suggest that it may have very high
mobility in soil.
Purification Methods
Crystallise it from n-butanol at -10o, collect it by vacuum filtration and wash it with cold diethyl ether. Harmful vapours. [Hurd Inorg Synth I 87 1939, Semon in Org Synth Coll Vol I 318 1932.]
히드록실아민 준비 용품 및 원자재
원자재
준비 용품