사프롤 C화학적 특성, 용도, 생산
개요
In the early 1990s, certain forest shrubs of the Piperaceae,
indigenous to the humid forests of Central America and
Greater Amazonia, were found to contain high levels of safrole
in their leaves. The Brazilian Amazon contains a wide variety of
Piper species but attention had focused on P. hispidinervum and
P. callosum, two species with high safrole content. Subsequently,
P. callosum has been dropped in the research work in
favor of the more promising P. hispidinervum. The essential oil
of P. hispidinervum contains high levels (83–93%) of safrole in
leaves, which can be easily extracted by hydrodistillation.
화학적 성질
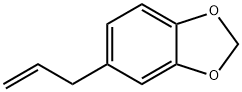
Safrole is a colorless to yellow liquid with an
odor of camphor or sassafras.
출처
Originally isolated in the oil from roots of Sassafras officinale; constituent of several essential oils, such as camphor, nutmeg and cinnamon leaves; the essential oil from the roots of Nemuaron humboldtii contains up to 99% safrole; Brazilian sassafras oil, up to 93%; and American sassafras oil, up to 80%. Also reported found in banana, cinnamon bark and leaf, nutmeg, mace, tamarind, pepper, cocoa, coriander seed, dill herb, dried bonito, lemon balm, ashanti pepper and green maté.
용도
Safrole, the main component of oil of sassafras, is widely used
as a flavoring agent in drugs and in the manufacture of heliotropin,
perfumes, soaps, and piperonyl butoxide (a compound
used in a variety of insecticides to enhance the pesticidal
properties of other active ingredients). Safrole has also been
used as a preservative in mucilage and library paste and as
a flotation frother. Oil of sassafras, which contains safrole, was
formerly used to flavor some soft drinks, such as root beer.
However, this was banned in the United States in 1960. Safrole
has also been used in the illicit production of the drug 3,4-
methylenedioxymethamphetamine (MDMA or ecstasy) and
the US Drug Enforcement Administration has designated
safrole a List I Chemical.
제조 방법
By distillation and/or freezing of such oils as Cinnamomum micranthum, Octea cymharum and oil of sassafras.
정의
ChEBI: A member of the class of benzodioxoles that is 1,3-benzodioxole which is substituted by an allyl group at position 5. It is found in several plants, including black pepper, cinnamon and nutmeg, and is present in several essential oils, notably that of sass
fras. It has insecticidal properties and has been used as a topical antiseptic. Although not thought to pose a significant carcinogenic risk to humans, findings of weak carcinogenicity in rats have resulted in the banning of its (previously widespread) use
in perfumes and soaps, and as a food additive.
일반 설명
Clear colorless or slightly yellow liquid with the odor of sassafras. Denser than water (density 1.09 g / cm3) and insoluble in water. Hence sinks in water. Obtained from oil of sassafras or oil of camphor.
공기와 물의 반응
Insoluble in water.
반응 프로필
Safrole, an acetal, is readily hydrolyzed in acidic solution to give 4-allylpyrocatechol and formaldehyde (or formaldehyde polymers).
위험도
Toxic by ingestion, may not be used in food
products (FDA), a possible carcinogen.
화재위험
Safrole is combustible.
Safety Profile
Confirmed carcinogen with experimental carcinogenic and neoplastigenic data. Poison by intravenous route. Moderately toxic by ingestion and subcutaneous routes. Experimental reproductive effects. Human mutation data
reported. A sktn irritant. Combustible when exposed to heat or flame. When heated to decomposition it emits acrid smoke and irritating fumes. See also ALDEHYDES and ALLYL COMPOUNDS.
Toxicology
Safrole is a colorless oily liquid possessing a
sweet, warm-spicy flavor. It has been used as a flavoring agent for more than 60 years. Oil of
sassafras, which contains 80% safrole, also has
been used as a spice. In the United States, the
FDA banned the use of safrole in 1958 and many other countries followed
this lead and also banned the use of safrole in flavors. Safrole, either naturally
occurring in sassafras oil or the synthetic chemical, has been shown
to induce liver tumors in rats. The continuous administration of safrole at
5,000 ppm in the total diet of rats caused liver tumors. Studies in dogs
showed extensive liver damage at 80 and 40 mg/kg, lesser damage at lower
levels, but no tumors. In vivo, safrole metabolites into 1’-hydroxysafrole.
The structures of safrole and 1’-hydroxysafrole are shown in Figure 10.14.
잠재적 노출
This compound has been used to
flavor beverages and foods. It is also reported to be used in
soap manufacture, perfumery, sleep aids, sedatives, and
pesticides. The FDA estimated exposure to safrole of the
general public through food consumption was extremely
low since the Agency prohibited its used in food. Derived
from oil of sassafras or camphor. Minimal exposure may
occur through the use of edible spices, including nutmeg
and mace, which contain low levels of naturally occurring
safrole.
Carcinogenicity
Safrole is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
신진 대사
Rats and guinea-pigs, dosed orally or ip with safrole, excreted in the urine 3-N,N-dimethylamino-1 -(3',4'-methylenedioxyphenyl)-1 -propanone (Oswald, Fishbein, Corbett & Walker, 1971). In addition, the rats excreted in the urine a major metabolite,3-piperidyl-l-(3',4'-methylenedioxyphenyl)-l-propanone, and traces of 3-pyrrolidinyl-l-(3',4'-methylenedioxyphenyl)-l-propanone. All three aminoketones decomposed to form 1-(3',4-methylenedioxyphenyl)-3-propen-l-one. Two metabolites formed by the epoxide-diol pathway and excreted in the urine of rats and guinea-pigs dosed with safrole were identified as l,2-methylenedioxy-4-(2',3'-dihydroxypropyl)benzene and l,2-dihydroxy-4-(2',3'-dihydroxypropyl)benzene (Horning, Bell, Carman & Stillwell, 1974).
운송 방법
Environmentally hazardous substances, liquid,
n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous
material, Technical Name Required.
Purification Methods
Safrole has been purified by fractional distillation, although it has also been recrystallised from low boiling pet ether at low temperatures. [IR: Briggs et al. Anal Chem 29 904 1957, UV: Patterson & Hibbert J Am Chem Soc 65 1962 1943.] The maleic anhydride adduct forms yellow crystals from toluene m 257o [Hickey J Org Chem 13 443 1948], and the picrate forms orange-red crystals from CHCl3 [Baril & Magrdichian J Am Chem Soc 58 1415 1936]. [Beilstein 19 H 39, 19 I 617, 19 II 29, 19 III/IV 275, 19/1 V 553.]
비 호환성
Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explo-
sions. Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides. Safrole, an acetal, is read-
ily hydrolyzed in acidic solution to give 4-allylpyrocatechol
and formaldehyde (or formaldehyde polymers)
폐기물 처리
Consult with environmental
regulatory agencies for guidance on acceptable disposal
practices. Generators of waste containing this contaminant
(≥100 kg/mo) must conform to EPA regulations governing
storage, transportation, treatment, and waste disposal. In
accordance with 40CFR165, follow recommendations for
the disposal of pesticides and pesticide containers. Must be
disposed properly by following package label directions or
by contacting your local or federal environmental control
agency, or by contacting your regional EPA office.
사프롤 준비 용품 및 원자재
원자재
준비 용품