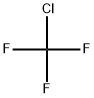
클로로트리플루오르메탄 C화학적 특성, 용도, 생산
개요
Strictly speaking, fluorocarbon compounds
contain only the elements carbon, fluorine, and
sometimes hydrogen. However, in industrial
applications such as refrigerants and aerosol
propellants, the term fluorocarbon has been
used to include compounds containing chlorine
and bromine atoms, or both. These industrial
products have somewhat similar chemical and
physical properties. Their relatively inert character
and wide range of vapor pressures and
boiling points make them especially well suited
as refrigerants in a variety of applications,
blowing agents for plastic foams, and aerosols.
화학적 성질
Colorless gas; ethereal odor. heavier than air. Nonflammable
용도
Dielectric and aerospace chemical, hardening
of metals, pharmaceutical processing.
일반 설명
CHLOROTRIFLUOROMETHANE is a colorless odorless gas. CHLOROTRIFLUOROMETHANE is shipped as a liquefied gas under its own vapor pressure. CHLOROTRIFLUOROMETHANE is noncombustible. CHLOROTRIFLUOROMETHANE can asphyxiate by the displacement of air. Contact with the liquid can cause frostbite. Exposure of the container to prolonged heat or fire may cause CHLOROTRIFLUOROMETHANE to rupture violently and rocket.
반응 프로필
The reaction of aluminum with various halogenated hydrocarbons produces a self-sustaining reaction with sufficient heat to melt aluminum pieces, examples of other halogenated hydrocarbons are fluorotrichloromethane, dichlorodifluoromethane, chlorodifluoromethane, tetrafluoromethane. The vigor of the reaction appears to be dependent on the combined degree of fluorination and the vapor pressure, [Chem. Eng. News 39(27):44(1961)].
위험도
Toxic by inhalation; slightly irritant.
건강위험
Exposure may cause nausea, dizziness, and headache, and rapid suffocation. Contact with skin may cause frostbite.
화재위험
Special Hazards of Combustion Products: Toxic fumes of Cl and F
Materials Uses
The fluorocarbons are generally compatible
with most of the common metals except at high
temperatures. At elevated temperatures, the
following metals resist fluorocarbon corrosion
(and are named in decreasing order of their corrosive
resistance): Inconel, stainless steel,
nickel, steel, and bronze. Water or water vapor
in fluorocarbon systems will corrode magnesium
alloys or aluminum containing over 2 percent
magnesium. These metals are not recommended
for use with fluorocarbon systems in
which water may be present.
Safety Profile
A mild irritant.
Narcotic in high concentrations. Reacts
violently with Al. When heated to
decomposition it emits highly toxic fumes of
Fand Cl-.
저장
Use forced ventilation and local exhaust, or
both, to prevent an accumulation of gas that
could reduce the oxygen level to below 19.5%.
Ensure good floor ventilation. Use a check
valve or trap in the discharge line to prevent
back flow into the cylinder. Where applicable,
use a pressure-reducing regulator when connecting
a cylinder to a low-pressure piping system.
For flammable fluorocarbons, adherence to
pertinent electrical standards is necessary. Personnel
should not weld, solder, braze, or have
an open flame of any type in atmospheres containing
flammable or nonflammable fluorocarbons.
Purification Methods
Main impurities are CO2, O2, and N2. The CO2 is removed by passage through saturated aqueous KOH, followed by conc H2SO4. The O2 is removed using a tower packed with activated copper on Kieselguhr at 200o, and the gas is dried over P2O5. [Miller & Smyth J Am Chem Soc 79 20 1957, Beilstein 1 III 42, 1 IV 34.] TOXIC GAS.
클로로트리플루오르메탄 준비 용품 및 원자재
원자재
준비 용품