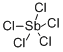안티몬 펜타염화물
|
|
안티몬 펜타염화물 속성
- 녹는점
- 2.8 °C(lit.)
- 끓는 점
- 92 °C30 mm Hg(lit.)
- 밀도
- 2.36 g/mL at 25 °C(lit.)
- 증기 밀도
- >10.2 (vs air)
- 증기압
- 0.13 psi ( 55 °C)
- 굴절률
- 1.601
- 인화점
- 79°C/22mm
- 용해도
- HCl, 클로로포름, 사염화탄소에 용해됩니다. 클로로포름, 염산, 타르타르산, 이황화탄소 및 사염화탄소와 혼합 가능합니다.
- 물리적 상태
- 액체
- Specific Gravity
- 2.336
- 색상
- 적황색
- 냄새
- 불쾌한 냄새
- 수용성
- 물(반응), 클로로포름, 염산 및 사염화탄소에 용해됩니다.
- 감도
- Moisture Sensitive
- Merck
- 14,695
- Dielectric constant
- 3.2200000000000002
- 안정성
- 안정적이지만 습기 및 물과 반응합니다. 화재가 발생하면 독성이 강한 연기를 방출합니다.
- CAS 데이터베이스
- 7647-18-9(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T,N,C | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 34-51/53-40-23/24/25-14-62-48/20-63 | ||
| 안전지침서 | 26-45-61-36/37/39-23 | ||
| 유엔번호(UN No.) | UN 1731 8/PG 3 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | CC5075000 | ||
| F 고인화성물질 | 10 | ||
| TSCA | Yes | ||
| 위험 등급 | 8 | ||
| 포장분류 | II | ||
| HS 번호 | 2827398520 | ||
| 유해 물질 데이터 | 7647-18-9(Hazardous Substances Data) | ||
| 독성 | LD50 orl-rat: 1115 mg/kg HYSAAV 29(12),16,64 | ||
| 기존화학 물질 | KE-01879 | ||
| 유해화학물질 필터링 | 97-1-176 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 무기 안티몬 화합물 및 이를 1% 이상 함유한 혼합물. 다만, 산화 안티몬(Antimony(Ⅴ) pentoxide, Antimony(Ⅳ) tetroxide), 황화안티몬(Antimony(Ⅴ) pentasulfide, Antimony(Ⅲ) trisulfide), 안티몬산(HSbO3) 염류(Antimonic(Ⅴ) acid, salts), 피그먼트 브라운 24(C.I. Pigment Brown 24), 피그먼트 옐로우 53 (C.I. Pigment Yellow 53) 및 이를 함유한 혼합물과 산화안티몬(Antimony(Ⅲ) trioxide)을 함유한 혼합물은 제외 |
안티몬 펜타염화물 C화학적 특성, 용도, 생산
화학적 성질
colourless to light yellow oily liquid물리적 성질
Antimony pentachloride is prepared by passing chlorine gas into molten antimony trichloride:SbCl3 + Cl2 →SbCl5
or by the reaction of the element with excess chlorine:
2 Sb + 5 Cl2 → 2 SbCl5.
용도
Antimony(V) chloride is used as a catalyst and an analytical reagent for testing alkaloids and cesium. It is also used as a polymerization catalyst as well as involved in the chlorination of organic compounds. It acts as a Lewis acid and a strong oxidizing agent.일반 설명
A reddish-yellow fuming liquid with a pungent odor. Fumes are irritating to the eyes and mucous membranes. Solidifies at 37°F. Corrosive to metals and tissue. Used to make other chemicals, and in chemical analysis.공기와 물의 반응
Fumes in air to form hydrochloric acid. Reacts with water to yield heat and antimony pentaoxide (Sb2O5) and hydrochloric acid [Merck 11th ed. 1989].반응 프로필
Acidic salts, such as ANTIMONY PENTACHLORIDE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.위험도
Corrosive, fumes in moist air, reacts strongly with organics.건강위험
Inhalation causes irritation of nose and throat. Contact of liquid with eyes or skin causes severe burns. Ingestion causes vomiting and severe burns of mouth and stomach. Overexposure by any route can cause bloody stools, slow pulse, low blood pressure, coma, convulsions, cardiac arrest.화재위험
Behavior in Fire: Irritating fumes of hydrogen chloride given off when water or foam is used to extinguish adjacent fire.Safety Profile
Poison by ingestion. Corrosive. Mutation data reported. See ANTIMONY COMPOUNDS and ANTIMONYPII) CHLORIDE. When heated to decomposition it emits very toxic fumes of Cland Sb.잠재적 노출
It is used in dyeing, coloring metals and in many organic chemical reactions as a catalyst.운송 방법
UN1730 (liquid) Antimony pentachloride, liquid, Hazard class: 8; Labels: 8-Corrosive material. UN1731 (solution) Antimony pentachloride, solutions, Hazard class: 8; Labels: 8-Corrosive material.비 호환성
Decomposes on contact with heat, acids, alkalis, ammonia, water or other forms of moisture producing fumes of hydrogen chloride and antimony. Decomposes above 77 C forming chlorine and antimony trichloride. Attacks many metals in the presence of moisture forming explosive hydrogen gas. Reacts with air forming corrosive vapors.폐기물 처리
Encapsulate and transfer to an approve landfill. If chemically treated and neutralized, the chemical is amenable to biological treatment at municipal sewage treatment plant. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/ mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.안티몬 펜타염화물 준비 용품 및 원자재
원자재
준비 용품
안티몬 펜타염화물 관련 검색:
삼염화안티몬 안티몬분 안티모니 트라이브로민화물 안티모니(III) 부톡시드(안티몬(III) 부톡시드) 흑연, 분말 옥시염화 안티모니(III) 글리신 안티몬 펜타염화물
TRIS(4-BROMOPHENYL)AMINIUM HEXACHLOROANTIMONATE
TRIPHENYLCARBENIUM HEXACHLOROANTIMONATE
TRIETHYLOXONIUM HEXACHLOROANTIMONATE
TRIMETHYLOXONIUM HEXACHLOROANTIMONATE
ANTIMONY, STANDARD SOLUTION 1000 MG/L SB FOR AA (ANTIMONY(III) CHLORIDE IN HYDROCHLORIC ACID 5 MOL/L)
ANTIMONY (III) CHLORIDE 99%
ANTIMONY(III) CHLORIDE REAGENT (FREE OF
ANTIMONY(III) CHLORIDE ACS REAGENT
NITROSONIUM HEXACHLOROANTIMONATE
METHYLOXOCARBENIUM(ACETYL)HEXACHLOROANTIMONATE