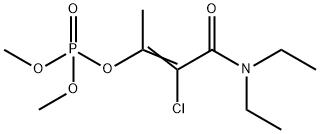
포스파미돈 C화학적 특성, 용도, 생산
개요
Types of phosphamidon formulations include soluble liquid, suspension concentrate and emulsifiable concentrate, ULV liquid, and 10% granules Phosphamidon is a pale yellow to colourless oily liquid with a faint odour. It is miscible with water and is soluble in aromatic hydrocarbons. Technical phosphamidon is a pale yellow to colourless oily liquid with a faint odour. It consists of a mixture of (Z)-isomer and (E)-isomer in the approximate proportion of 70:30. It decomposes on heating and releases highly toxic fumes such as phosphorus oxides, hydrogen chloride, and nitrogen oxides. Phosphamidon reacts and gets rapidly hydrolysed by alkalis and decomposes on
heating or on burning, producing highly toxic fumes. It attacks metals such as iron, tin,
and aluminium. It
is used as a broad-spectrum insecticide and acaricide for the control of pests and vectors
on crops like sugarcane, rice, citrus orchards, and cotton.
화학적 성질
Phosphamidon is a pale yellow to colorless oily liquid with a faint odor. It is miscible
with water and is soluble in aromatic hydrocarbons. Phosphamidon decomposes on
heating and releases highly toxic fumes, such as phosphorus oxides, hydrogen chloride,
and nitrogen oxides. It reacts with bases (hydrolysis) and attacks metals such as iron,
tin, and aluminium. Phosphamidon should be handled by trained personnel wearing
protective clothing. Phosphamidon is used as a broad-spectrum insecticide and acaricide
for the control of pests and vectors on crops like sugar cane, rice, citrus orchards,
and cotton. Occupational exposures to phosphamidon occur among factory workers
involved in synthesizing formulation and dispensing spray operations. Human exposures
also occur among crop harvesters and in vector control operations.
용도
Phosphamidon is used to control sucking and boring insects and
mites in a very wide range of crops and in forestry applications.
일반 설명
Pale yellow oily liquid with a faint odor. Used as an insecticide for citrus, cotton, and deciduous fruit and nuts. and as an acaricide.
공기와 물의 반응
Water soluble. Hydrolyzed by alkali with a half-life at 73°F of 13.8 days at pH 7 and 2.2 days at pH 10 .
반응 프로필
PHOSPHAMIDON is corrosive to iron, tin and aluminum. Incompatible with alkaline preparations and should not be mixed with copper oxychloride, captan, folpet or sulfur.
건강위험
PHOSPHAMIDON is extremely toxic; the probable oral lethal dose for humans is 5-50 mg/kg, or between 7 drops and 1 teaspoonful for a 150-lb person. It is a cholinesterase inhibitor.
화재위험
(Non-Specific -- Organophosphorus Pesticide, Liquid, n.o.s.) Container may explode in heat of fire. Heat above 320F may cause decomposition and evolution of highly toxic fumes of phosphorus oxides and chlorides. Hydrolyzes in alkali. Stable in neutral and acid media. Hydrolyzes in alkali.
잠재적 노출
This material is used as an insecticide on citrus, cotton, and deciduous fruit and nuts. It is also an acaricide.
환경귀착
Chemical/Physical. Emits toxic fumes of chlorine, phosphorus and nitrogen oxides
when heated to decomposition (Sax and Lewis, 1987)
신진 대사 경로
The metabolism of phosphamidon has been reviewed by Geissbuhler et al.
(1971) and Beynon et al. (1973). Technical phosphamidon consists of two
stereochemical isomers in the E:Z ratio of ca. 3:7. The Z-isomer has the
greater insecticidal activity. It is important to note that in the case of
phosphamidon the isomer with the phosphate ester function trans to
the amide group is assigned the Z configuration due to the priority of
chlorine, whereas in the case of mevinphos, monocrotophos and dicrotophos
it is assigned the E configuration. In all four compounds, the isomers
with this configuration (also referred to as the cis-crotonamide or crotonate
structure) have the greater insecticidal activities. It is a systemic
insecticide which is rapidly translocated in the plant via the xylem. Phosphamidon
is rapidly degraded in the environment, the major routes being
via N-de-ethylation and cleavage of the P-O-vinyl function. The resultant
N,N-diethyl-2-chloroacetoacetamide or N-ethyl-2-chloroacetoacetamide
are then degraded via dechlorination and hydrolysis, ultimately to give
acetone, diethylamine and ethylamine. Conjugated metabolites have not
been identified.
신진 대사
The major routes of degradation are oxidative
dealkylation of the amide group and hydrolysis of the vinyl
phosphate ester bond. Dechlorination also occurs. In soils,
DT
50 is 7–25 d depending upon the soil type.
운송 방법
UN3018 Organophosphorus pesticides, liquid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
비 호환성
Compounds of the carboxyl group react with all bases, both inorganic and organic (i.e., amines) releasing substantial heat, water and a salt that may be harmful. Incompatible with arsenic compounds (releases hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides (releasing heat, toxic and possibly flammable gases), thiosulfates and dithionites (releasing hydrogen sulfate and oxides of sulfur). Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Strong oxidizers may cause release of toxic phosphorus oxides. Organophosphates, in the presence of strong reducing agents such as hydrides, may form highly toxic and flammable phosphine gas. Keep away from alkaline materials. Attacks metals, such as aluminum, iron, tin.
폐기물 처리
Small quantities may be treated with alkali followed by landfill disposal. Large quantities should be incinerated with effluent gas scrubbing. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
포스파미돈 준비 용품 및 원자재
원자재
준비 용품