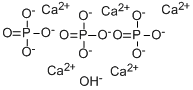트리염기 칼슘 인산염
|
|
트리염기 칼슘 인산염 속성
- 녹는점
- 1100 °C(lit.)
- 밀도
- 1.038 g/mL at 25 °C
- 저장 조건
- 2-8°C
- 용해도
- 1M 염산에 용해됩니다.
- 물리적 상태
- 수성 현탁액
- 색상
- 하얀색
- 수소이온지수(pH)
- 4-6
- 수용성
- 물에 부분적으로 용해됩니다.
- Merck
- 1694
- CAS 데이터베이스
- 12167-74-7(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/37/38 | ||
| 안전지침서 | 26-36-24/25-22 | ||
| WGK 독일 | 3 | ||
| TSCA | Yes | ||
| HS 번호 | 2835260000 | ||
| 기존화학 물질 | KE-04599 |
트리염기 칼슘 인산염 C화학적 특성, 용도, 생산
화학적 성질
The PhEur 6.4 states that tribasic calcium phosphate consists of a mixture of calcium phosphates. It contains not less than 35.0% and not more than the equivalent of 40.0% of calcium. The USP32– NF27 specifies that tribasic calcium phosphate consists of variable mixtures of calcium phosphates having the approximate composition 10CaO·3P2O5·H2O. This corresponds to a molecular formula of Ca5(OH)(PO4)3 or Ca10(OH)2(PO4)6.Tribasic calcium phosphate is a white, odorless and tasteless powder. col hexagonal crystal(s) [CRC10]
용도
Calcium phosphate tribasic (C3161) is plant cell culture tested (0.2 mg/ml) and is appropriate for use in plant cell culture experiments. Calcium phosphate tribasic is utilized to engineer new biomaterials for applications such as bone grafts and fillers.주요 응용
Calcium phosphate tribasic is the mineral component of bones and teeth; it has been used therapeutically as a prosthetic aid and in the prevention and treatment of osteoporosis. Employed in tissue replacement for repairing bony defects and acts as drug carrier for drug delivery to the bone. Hydroxyapatite nanopowders, incorporated in biodegradable polymer composites or deposited on biocompatible substrates, have been shown to promote adhesions and proliferation of bone-forming (osteoblast) cells. It is also the starting material for the production of phosphoric acid and fertilizers.생산 방법
Tribasic calcium phosphate occurs naturally as the minerals hydroxylapatite, voelicherite, and whitlockite. Commercially, it is prepared by treating phosphate-containing rock with sulfuric acid. Tribasic calcium phosphate powder is then precipitated by the addition of calcium hydroxide. Tribasic calcium phosphate is alternatively prepared by treating calcium hydroxide from limestone with purified phosphoric acid. It may also be obtained from calcined animal bones. Some tribasic calcium phosphate products may be prepared in coarser, directly compressible forms by granulating the powder using roller compaction or spray drying.일반 설명
Hydroxyapatite is a key inorganic phosphate that has chemical and structural resemblance to bone. HAp is a popular biomaterial which finds use as a bone or teeth replacement and repair, hard tissue repair.Pharmaceutical Applications
Tribasic calcium phosphate is widely used as a capsule diluent and tablet filler/binder in either direct-compression or wet-granulation processes. The primary bonding mechanism in compaction is plastic deformation. As with dibasic calcium phosphate, a lubricant and a disintegrant should usually be incorporated in capsule or tablet formulations that include tribasic calcium phosphate. In some cases tribasic calcium phosphate has been used as a disintegrant.It is most widely used in vitamin and mineral preparations as a filler and as a binder. It is a source of both calcium and phosphorus, the two main osteogenic minerals for bone health. The bioavailability of the calcium is well known to be improved by the presence of cholecalciferol. Recent research reports that combinations of tribasic calcium phosphate and vitamin D3 are a cost-effective advance in bone fracture prevention.In food applications, tribasic calcium phosphate powder is widely used as an anticaking agent.
Safety
Tribasic calcium phosphate is widely used in oral pharmaceutical formulations and food products, and is generally regarded as nontoxic and nonirritant at the levels employed as a pharmaceutical excipient.Ingestion or inhalation of excessive quantities may result in the deposition of tribasic calcium phosphate crystals in tissues. These crystals may lead to inflammation and cause tissue lesions in the areas of deposition.
Oral ingestion of very large quantities of tribasic calcium phosphate may cause abdominal discomfort such as nausea and vomiting.
No teratogenic effects were found in chicken embryos exposed to a dose of 2.5 mg of tribasic calcium phosphate.
LD50 (rat, oral): >1 g/kg
저장
Tribasic calcium phosphate is a chemically stable material, and is also not liable to cake during storage.The bulk material should be stored in a well-closed container in a cool, dry place.
비 호환성
All calcium salts are incompatible with tetracycline antibiotics. Tribasic calcium phosphate is incompatible with tocopheryl acetate (but not tocopheryl succinate). Tribasic calcium phosphate may form sparingly soluble phosphates with hormones.Regulatory Status
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (oral capsules and tablets). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.트리염기 칼슘 인산염 준비 용품 및 원자재
원자재
준비 용품
트리염기 칼슘 인산염 공급 업체
글로벌( 182)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 |
hbjbtech@163.com | China | 1000 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |
| Zhengzhou Alfa Chemical Co.,Ltd | +8618530059196 |
sale04@alfachem.cn | China | 12468 | 58 |
| SIMAGCHEM CORP | +86-13806087780 |
sale@simagchem.com | China | 17367 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 |
marketing@targetmol.com | United States | 19892 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 |
sales@tnjchem.com | China | 34572 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 |
1026@dideu.com | China | 9320 | 58 |