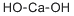수산화칼슘
|
|
수산화칼슘 속성
- 녹는점
- 580 °C
- 끓는 점
- 2850 °C
- 밀도
- 2.24 g/mL at 25 °C (lit.)
- 저장 조건
- Store at +5°C to +30°C.
- 용해도
- 1.7g/L
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 산도 계수 (pKa)
- 12.6[at 20 ℃]
- 색상
- 하얀색
- 냄새
- 냄새 없는
- pH 범위
- 12.4
- 수소이온지수(pH)
- 11.27(1 mM solution);12.2(10 mM solution);12.46(100 mM solution);
- 수용성
- 1.65g/L(20℃)
- 감도
- Air Sensitive
- Merck
- 14,1673
- Solubility Product Constant (Ksp)
- pKsp: 5.26
- 노출 한도
- ACGIH: TWA 5 mg/m3
OSHA: TWA 15 mg/m3; TWA 5 mg/m3
NIOSH: TWA 5 mg/m3
- 안정성
- 안정적인. 강산과 호환되지 않습니다.
- CAS 데이터베이스
- 1305-62-0(CAS DataBase Reference)
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi,C | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 41-34-37/38 | ||
| 안전지침서 | 26-39-45-36/37/39-27 | ||
| 유엔번호(UN No.) | 3262 | ||
| OEB | B | ||
| OEL | TWA: 5 mg/m3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | EW2800000 | ||
| F 고인화성물질 | 34 | ||
| TSCA | Yes | ||
| HS 번호 | 2825 90 19 | ||
| 위험 등급 | 8 | ||
| 포장분류 | III | ||
| 유해 물질 데이터 | 1305-62-0(Hazardous Substances Data) | ||
| 독성 | LD50 orally in rats: 7.34 g/kg (Smyth) | ||
| 기존화학 물질 | KE-04518 |
수산화칼슘 C화학적 특성, 용도, 생산
순도시험
(1) 염산불용물 : 이 품목 2g에 염산 10mL 및 물 20mL를 가하여 끓이고 식힌 다음 정량분석용여과지로 여과하여 여과지상의 잔류물을 씻은 액이 염화물의 반응을 나타내지 아니할 때까지 열탕으로 잘 씻은 다음 여과지와 함께 회화하였을 때, 그 양은 10mg 이하이어야 한다.
(2) 탄산염 : 이 품목 2g에 물 50mL를 가하여 잘 흔들어 섞은 다음 묽은염산 25mL를 가할 때, 심한 거품이 일어나서는 아니 된다.
(3) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 4.0ppm 이하이어야 한다.
(4) 납 : 「메타인산나트륨」의 순도시험 (2)에 따라 시험한다(2.0ppm 이하).
(5) 불소화물 : 이 품목 1g을 정밀히 달아 「구연산칼슘」의 순도시험 (8)에 따라 시험한다(50ppm 이하).
(6) 알칼리금속 및 마그네슘 : 이 품목 0.5g에 1N 염산 30mL를 가하여 필요하면 수욕 중에서 가열하여 녹이고 식힌 다음 암모니아시액으로 중화하고 수산암모늄시액 30mL를 가하여 수욕상에서 1시간 가열한다. 식힌 다음 물을 가하여 100mL로 하고 잘 저어 섞은 다음 여과하여 여액 50mL를 취하여 이에 황산 0.5mL를 가하여 증발건고한 다음 항량이 될 때까지 강열할 때, 그 양은 12mg 이하이어야 한다.
(7) 바륨 : 이 품목 1.5g을 묽은염산 15mL에 녹여 물을 가하여 30mL로 하고 여과한 여액 20mL를 취하여 초산나트륨 2g, 묽은초산 1mL 및 크롬산칼륨시액 0.5mL를 가하여 15분간 방치할 때, 그 액의 탁도는 바륨표준용액 0.3mL를 취해서 물을 가하여 20mL로 한 액에 대하여 위와 같이 조작을 하였을 때의 탁도 이하이어야 한다(0.03% 이하).
확인시험
(1) 이 품목에 3~4배량의 물을 가하면 죽모양으로 되고 알칼리성을 나타낸다.
(2) 이 품목 1g에 물 20mL 및 초산 6mL를 가하여 녹인 액은 확인시험법 중 칼슘염의 반응을 나타낸다.
정량법
이 품목 약 2g을 정밀히 달아 묽은염산 30mL에 녹이고 물을 가하여 250mL로 한다. 그 중 10mL에 수산화칼륨용액(1→10) 15mL, 시안화칼륨용액(1→20) 3mL 및 물 100mL를 가하여 약 1분간 방치하고 2-옥시-1-(2''-옥시-4''-설포-1''-나프틸아조)-3-나프토에산시약 0.1g을 가해주고 즉시 0.05M 이.디.티.에이.용액으로 적정한다. 종말점은 적색이 완전히 소실되고 청색으로 된 때로 한다.
0.05M 이.디.티.에이.용액 1mL = 3.705mg Ca(OH)2
개요
Calcium Hydroxide is a colorless crystal or white powder, obtained when CaO (called lime or quicklime) is mixed, or “slaked” with water. It can also be precipitated by mixing CaCl2 andNaOH.The nameof the natural,mineral formis “portlandite”. It is a relatively rare mineral, known from some volcanic, plutonic, and metamorphic rocks. It has also been known to be formed in burning coal dumps.화학적 성질
Calcium hydroxide occurs as a white or almost white, crystalline or granular powder. It has a bitter, alkaline taste. Calcium hydroxide readily absorbs carbon dioxide to form calcium carbonate.물리적 성질
Soft white crystalline powder; hexagonal; density 2.34 g/cm3; slightly bitter taste; loses water when heated at elevated temperatures (580°C); slightly soluble in water; Ksp 1.2x10-14; aqueous solution alkaline; soluble in glycerol and acids; insoluble in alcohol.용도
calcium hydroxide is an inorganic base used to adjust the pH of a product, it can also serve as a topical astringent and alkali in solutions or lotions. It can burn the skin and eyes.정의
Lime water: A solution of calcium hydroxide in water. If carbon dioxide is bubbled through lime water a milky precipitate of calcium carbonate forms. Prolonged bubbling of carbon dioxide turns the solution clear again as a result of the formation of soluble calcium hydrogencarbonate (Ca(HCO3)2).생산 방법
Calcium hydroxide is manufactured by adding water to calcium oxide, a process called slaking.일반 설명
Odorless white granules. Sinks in water.공기와 물의 반응
Water soluble. The amount of heat generated by hydrolysis may be large.반응 프로필
The nitroparaffins, nitromethane, nitropropane, etc. form salts with inorganic bases such as Calcium hydroxide . The dry salts are explosive [Chem. Eng. news 30:2344 1952]. Bases are chemically similar to sodium hydroxide (NaOH) or sodium oxide (Na2O). They neutralize acids exothermically to form salts plus water. When soluble in water they give solutions having a pH greater than 7.0. Mixing these materials with water can generate troublesome amounts of heat as the base is dissolved or diluted. Bases react with certain metals (such as aluminum and zinc) to form oxides or hydroxides of the metal and generate gaseous hydrogen. Bases may initiate polymerization reactions in polymerizable organic compounds, especially epoxides). They may generate flammable and/or toxic gases with ammonium salts, nitrides, halogenated organics, various metals, peroxides, and hydroperoxides. Materials of this group often serve as catalysts. A strong base. Forms caustic solution in water [Merck 11th ed. 1989].위험도
Skin, eye, and upper respiratory tract irri- tant, avoid inhalation.건강위험
Dust irritates eyes, nose and throat.Pharmaceutical Applications
Calcium hydroxide is a strong alkali and is used as a pharmaceutical pH adjuster/buffer and antacid in topical medicinal ointments, creams, lotions, and suspensions, often as an aqueous solution (lime water). It forms calcium soaps of fatty acids, which produce water-in-oil emulsions (calamine liniment), and it is also used as a topical astringent.Calcium hydroxide is a common cosmetic ingredient in hairstraightening and hair-removal products, and in shaving preparations.
1) In dentistry, it is used as a filling agent and in dental pastes to encourage deposition of secondary dentine. Calcium hydroxide was traditionally used as an escharotic in Vienna Paste.
농업용
Calcium hydroxide, [Ca(OH)2], also called slaked lime or hydrated lime, is a white solid that dissolves sparingly in water. It is manufactured by adding water to calcium oxide, a process that emits heat and is known as slaking.Calcium hydroxide is a cheap alkali, used for neutralizing the acidity of acid soils and in the manufacture of mortar, white wash, bleaching powder and glass. It is an excellent absorbent for carbon dioxide to produce insoluble calcium carbonate and has a neutralizing value of 179%, compared to 100% of pure calcium carbonate.
Safety Profile
Mildly toxic by ingestion. A severe eye irritant. A skin, mucous membrane, and respiratory system irritant. Mutation data reported. Causes dermatitis. Dust is considered to be a significant industrial hazard. A common air contaminant. Violent reaction with maleic anhydride, nitroethane, nitromethane, nitroparaffins, nitropropane, phosphorus. Reaction with polychloiinated phenols + potassium nitrate forms extremely toxic products. See also CALCIUM COMPOUNDSSafety
Calcium hydroxide is used in oral and topical pharmaceutical formulations. It is mildly toxic by ingestion. In the pure state, calcium hydroxide is a severe skin, eye, and respiratory irritant, and it is corrosive, causing burns. Typical exposure limits are TVL 5 mg/m3 in air.LD50 (mouse, oral): 7.3 g/kg
LD50 (rat, oral): 7.34 g/kg
잠재적 노출
Calcium hydroxide is used in agriculture and in fertilizer manufacture; it is used in the formulation of mortar, plasters, and cements; it is used as a scrubbing and neutralizing agent in the chemical industry. For making insecticides, acaricides, and products to control arthropods저장
Calcium hydroxide should be stored in an airtight container, in a cool, dry, well-ventilated place. Calcium hydroxide powder may be sterilized by heating for 1 hour at a temperature of at least 1608℃.운송 방법
UN3262 Corrosive solid, basic, inorganic, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name requiredPurification Methods
Heat analytical grade calcium carbonate at 1000o during 1hour. Allow the resulting oxide to cool and add slowly to water. Heat the suspension to boiling, cool and filter through a sintered glass funnel of medium porosity (to remove soluble alkaline impurities). Dry the solid at 110o and crush it to a uniformly fine powder. [Ehrlich in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 934 1963.]비 호환성
Incompatible with strong acids, maleic anhydride, phosphorus, nitroethane, nitromethane, nitroparaffins, and nitropropane. Calcium hydroxide can be corrosive to some metals.폐기물 처리
Landfill or admixture with acid industrial wastes prior to lagooningRegulatory Status
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (intravenous and subcutaneous injections; oral suspensions and tablets; topical emulsions and creams). Included in parenteral preparations licensed in the UK.수산화칼슘 준비 용품 및 원자재
원자재
준비 용품
수산화칼슘 공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 |
1022@dideu.com | China | 3878 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 20291 | 58 |
| Yujiang Chemical (Shandong) Co.,Ltd. | +86-17736087130 +86-18633844644 |
catherine@yjchem.com.cn | China | 983 | 58 |
| Hebei Youfeidi Trading Co., LTD | +8615530197691 |
admin@yfdchem.cn | China | 105 | 58 |
| Jiangsu Kolod Food Ingredients Co.,Ltd. | +86-518-85110578 +8618805133257 |
sales3257@jskolod.com | China | 132 | 60 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 |
info@tnjchem.com | China | 2989 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 |
zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29888 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8820 | 58 |