|
|
| | (1S, 2S)-(1-BENZYL-3-CHLORO-2-HYDROXY-PROPYL)-CARBAMIC ACID TERT-BUTYL ESTER Basic information | | Application |
| Product Name: | (1S, 2S)-(1-BENZYL-3-CHLORO-2-HYDROXY-PROPYL)-CARBAMIC ACID TERT-BUTYL ESTER | | Synonyms: | (1S, 2S)-(1-BENZYL-3-CHLORO-2-HYDROXY-PROPYL)-CARBAMIC ACID TERT-BUTYL ESTER;(1S,2S)-[3-CHLORO-2-HYDROXY-1-(PHENYLMETHYL)PROPYL]CARBAMIC ACID 1,1-DIMETHYLETHYL ESTER;(1S, 2S)-[3-CHLORO-2-HYDROXY-1-(PHENYLMETHYL)-PROPYL]CARBAMIC ACID, 1,1-DIMETHYLETHYL ETHER;(2S,3S)-(-)-3-T-BUTOXYCARBONYLAMINO-1-CHLORO-4-PHENYL-BUTAN-2-OL;(2S,3S)-3-(tert-Butoxycarbonylamino)-1-chloro-2-hydroxy-4-phenylbutane;(1S, 2S)-[3-Chloro-2-hydroxy-1-(phenylmethyl)-;Tert-Butyl (2S,3S)-4-chloro-3-hydroxy-1-phenylbutan-2-ylcarbamate;Carbamic acid, N-[(1S,2S)-3-chloro-2-hydroxy-1-(phenylmethyl)propyl]-, 1,1-dimethylethyl ester | | CAS: | 165727-45-7 | | MF: | C15H22ClNO3 | | MW: | 299.79 | | EINECS: | 1312995-182-4 | | Product Categories: | | | Mol File: | 165727-45-7.mol | 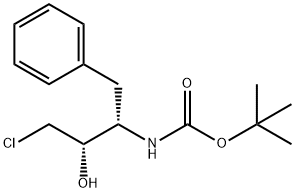 |
| | (1S, 2S)-(1-BENZYL-3-CHLORO-2-HYDROXY-PROPYL)-CARBAMIC ACID TERT-BUTYL ESTER Chemical Properties |
| Boiling point | 460.5±45.0 °C(Predicted) | | density | 1.153±0.06 g/cm3(Predicted) | | storage temp. | 2-8°C | | pka | 11.92±0.46(Predicted) | | Appearance | White to off-white Solid | | Optical Rotation | Consistent with structure | | CAS DataBase Reference | 165727-45-7(CAS DataBase Reference) |
| | (1S, 2S)-(1-BENZYL-3-CHLORO-2-HYDROXY-PROPYL)-CARBAMIC ACID TERT-BUTYL ESTER Usage And Synthesis |
| Application | (1S,2S)-(1-benzyl-3-chloro-2-hydroxypropyl) tert-butyl carbamate is mainly used as an intermediate in the active pharmaceutical ingredient darunavir, primarily in laboratory research and development processes and pharmaceutical and chemical production processes. | | Synthesis | Using (3S)-3-(tert-butoxycarbonyl)amino-1-chloro-4-phenyl-2-butanone as starting material, the MPV reduction reaction was carried out in different organic solvents according to the methodology described in Example 3, where the target solvent was substituted for toluene. Isopropanol (10%) was added to each solvent to ensure that the reaction was completed in a suitable time. Table 1 shows the results of these reactions. The (R,S)/(S,S) ratios increased when the reactions were carried out in nonproton polar solvents such as ethyl acetate and THF. Although not subject to any theoretical constraints, it is hypothesized that the hydrogen bonding between ketone 1 and Al(OiPr)3 may facilitate the reaction rate by maintaining the coordination state of 1 with the aluminum center, thus bringing the two reaction centers closer together. Table 1 lists the results of MPV reduction of 1 in different organic solvents. | | References | [1] Patent: WO2011/60302, 2011, A1. Location in patent: Page/Page column 20
[2] Patent: CN104387299, 2016, B. Location in patent: Paragraph 0082; 0083
[3] Patent: US2002/151722, 2002, A1
[4] Tetrahedron Asymmetry, 1997, vol. 8, # 15, p. 2547 - 2552
[5] Patent: US5936104, 1999, A |
| | (1S, 2S)-(1-BENZYL-3-CHLORO-2-HYDROXY-PROPYL)-CARBAMIC ACID TERT-BUTYL ESTER Preparation Products And Raw materials |
|