- etravirine
-

- $1.00 / 1mg
-
2024-04-15
- CAS:269055-15-4
- Min. Order: 1mg
- Purity: 0.99
- Supply Ability: 5ton/month
- etravirine
-

- $4.00 / 1KG
-
2024-01-08
- CAS:269055-15-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000tons
- Etravirine
-

- $50.00 / 1kg
-
2023-09-06
- CAS:269055-15-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10000tons
Related articles - Uses and side effects of Etravirine
- Etravirine is an antiretroviral agent more specifically classified as a Non-Nucleoside Reverse Transcriptase Inhibitor (NNRTI)....
- Apr 19,2022
|
| | Etravirine Basic information |
| | Etravirine Chemical Properties |
| Melting point | .265°C (dec.) | | Boiling point | 637.4±65.0 °C(Predicted) | | density | 1.439 g/cm3 | | storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C | | solubility | DMSO (Slightly), Methanol (Slightly, Heated, Sonicated) | | form | Solid | | pka | 1.23±0.10(Predicted) | | color | White to Off-White |
| | Etravirine Usage And Synthesis |
| Description | Etravirine is a second-generation NNRTI. It is indicated for use in
combination with other antiretroviral agents for treating HIV-1 infection in
treatment-experienced adult patients who have evidence of viral replication and HIV-1 strains resistant to the currently available NNRTIs and
other antiretroviral agents. The NNRTIs, along with nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs/NtRTIs), are important
components of the combination regimens currently used to treat HIV-1
infection. The NRTIs/NtRTIs act by competing with the natural nucleotide
substrates of reverse transcriptase for incorporation into viral DNA
and subsequent chain termination. By contrast, the NNRTIs bind to an
allosteric site of the enzyme and disrupt the DNA polymerase function by
inducing conformational changes to the catalytic site. The allosteric
binding nature of NNRTIs generally results in improved safety profile
since there is no known human homolog for the drug-binding site of
the enzyme.
As with other NNRTIs, etravirine has
many drug–drug interactions. It is a substrate of CYP3A4, CYP2C9,
and CYP2C19, an inducer of 3A4, and an inhibitor of 2C9 and 2C19.
Caution should be used with co-administration of inducers, inhibitors, or
substrates of these enzymes. Etravirine can be synthesized starting from
5-bromo-2,4,6-trichloropyrimidine through three successive nucleophilic
substitution reactions. Initial displacement with 4-aminobenzonitrile using
Hu¨nig’s base, followed by reaction with sodium salt of 4-hydroxy-3,5-
dimethylbenzonitrile, and subsequent ammonolysis reaction with ammonia in dioxane under pressure affords etravirine.
. | | Chemical Properties | White to Off-White Solid | | Originator | Janssen (United States) | | Uses | Etravirine is a novel HIV reverse transcriptase inhibitor useful in treatment of HIV infection. | | Uses | Etravirine (TMC125) is a non-nucleoside reverse transcriptase inhibitor (NNRTI) used for the treatment of HIV. | | Uses | Etravirine is an antiretroviral (anti-HIV) drug that is part of the non nucleoside reverse transcriptase inhibitor (NNRTIs or Non Nukes) family. It is used together with other antiretrovirals in treatment-experienced adult patients, who have failed previous therapy, and have HIV-1 strains which are resistant to other retrovirals and NNRTIs. Etravirine is used to delay the progression of HIV infection. By using etravirine, your immune system should improve (increase in CD4 + count) and you will be better protected against opportunistic infections.
Etravirine does not cure AIDS or completely kill the HIV virus, but helps to prevent further damage by slowing down the production of new viruses. Treatment with etravirine does not reduce the risk of passing infection on to others. You will still be able to pass HIV by sexual contact, by blood transfer or by sharing needles. You should always use appropriate precautions to prevent passing HIV on to others. | | Definition | ChEBI: An aminopyrimidine that consists of 2,6-diaminopyrimidine bearing a bromo substituent at position 5, a 4-cyano-2,6-dimethylphenoxy substituent at position 4 and having a 4-cyanophenyl substituent attached to the 2-amino group. NNRTI of HIV-1, binds directl
to RT and blocks RNA-dependent and DNA-dependent DNA polymerase activities | | Brand name | Intelence | | Acquired resistance | Various mutations are associated with a decreased virological
response. Single codon substitutions at positions 100, 101
and 181 are considered major mutations. A single K103N
mutation is not associated with resistance. | | Pharmaceutical Applications | A comprehensive analysis of baseline resistance data from
the DUET-1 and DUET-2 studies has identified a list of
17 etravirine resistance associated mutations: V901, A98G,
L100L, K101E/H/I, V1061, E138A, V179D/F/T, Y181C/L/V,
G190A/S, and M230L. A single K103N mutation is not associated
with resistance to etravirine. | | Mechanism of action | Etravirine binds directly to reverse transcriptase and blocks the RNA-dependent and DNA-dependent DNA polymerase activities by causing a disruption of the enzyme's catalytic site. Etravirine does not inhibit the human DNA polymerases alpha, beta, and gamma. | | Pharmacokinetics | Oral absorption: Not known/available
Cmax 200 mg oral twice daily: c. 959 ng/mL
Cmin 200 mg oral twice daily: c. 469 ng/mL
Plasma half-life: c. 36 h
Volume of distribution: Not known/available
Plasma protein binding: >99%
Administration with food improves the bioavailability and
reduces interpatient variability. It undergoes oxidative metabolism
by cytochrome P450 systems. Around 93.7% and 1.2%
of an administered dose can be retrieved in the feces and
urine, respectively, mostly as unchanged drug.
Details of distribution into CSF, semen and breast milk
and recommendations for dose adjustment in patients with
hepatic impairment are not yet available. | | Clinical Use | Treatment of HIV-1 infection in adults (in combination with other
antiretroviral drugs) | | Side effects | In the phase III studies around 15% of patients experienced
erythematous or maculopapular rashes of mild or moderate
severity; most resolved with continued dosing, but treatment
was discontinued in 2% of patients. Rare cases of Stevens–
Johnson syndrome have been reported.
Other common adverse events are diarrhea, nausea, headache
and fatigue. Dyslipidemia and raised pancreatic amylase
occur in some patients. | | Synthesis | Only the discovery
synthesis and small scale syntheses
have been disclosed for this compound in the following scheme. The
largest scale synthesis was initiated by the portionwise addition
of cyanamide to a solution of the p-cyanoaniline hydrochloride
salt 73. The mixture was refluxed in diglyme to give
guanidine salt 74 in 85% yield after concentration of the reaction
mixture and recrystallization from acetone. Reaction
of guanidine 74 with diethylmalonate in the presence of
sodium ethoxide in refluxing ethanol gave pyrimidine diol
75 in 57% yield, which upon refluxing in phosphorous oxychloride
for 30 min gave dichloride 76 in 97% yield. Bromination
of dichloride 76 with NBS in chloroform at room
temperature provided bromide 77 in 55% yield. Heating a
mixture of the dichlorobromide 77 with the sodium salt of
2,5-dimethyl-4-cyanophenol 78, generated by reaction with
sodium hydride in situ) in diglyme and NMP at 155 ??C gave
the coupled product 79 in 45% yield. Finally, reaction of the
chloride 79 with ammonia in refluxing dioxane (or iPrOH) in
a sealed tube gave etravirine (IX) in 41% yield after purification. 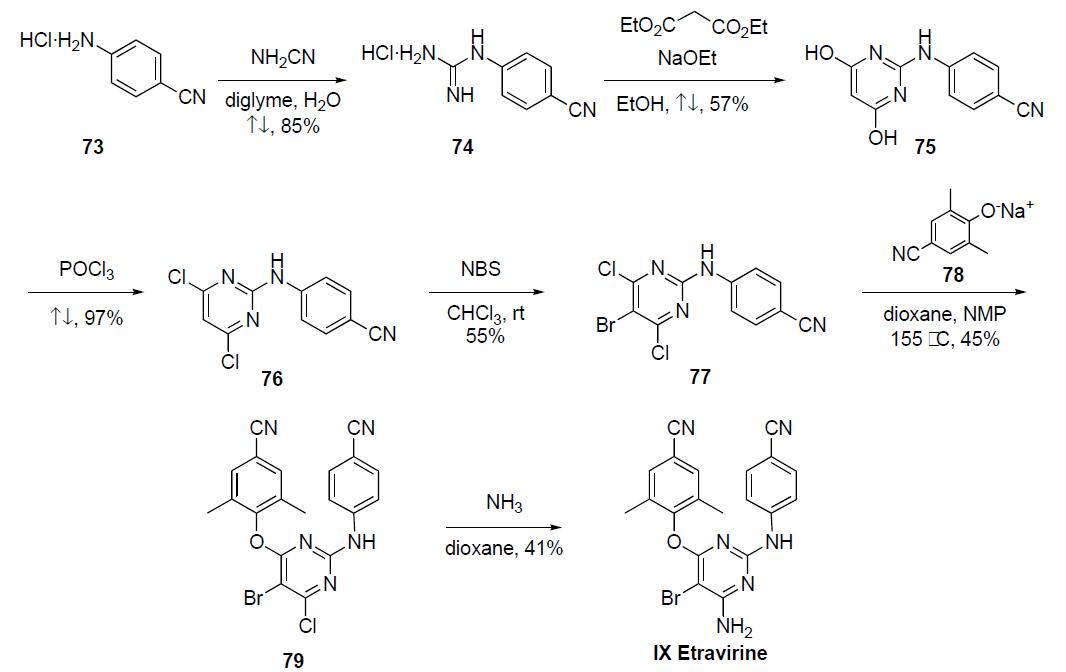
| | Drug interactions | Potentially hazardous interactions with other drugs
Antibacterials: concentration increased by
clarithromycin, also concentration of clarithromycin
reduced; concentration of both drugs reduced with
rifabutin; avoid concomitant use with rifampicin.
Antivirals: concentration possibly reduced by
efavirenz and nevirapine - avoid concomitant use;
concentration of fosamprenavir increased, consider
reducing fosamprenavir dose; possibly reduces
bosutinib and indinavir concentration - avoid
concomitant use; concentration of dolutegravir
reduced; possibly reduces concentration of
maraviroc; concentration reduced by tipranavir
and tipranavir concentration increased - avoid
concomitant use.
Clopidogrel: possibly reduced antiplatelet effect.
Guanfacine: possibly reduces concentration of
guanfacine - increase guanfacine dose.
Orlistat: absorption possibly reduced by orlistat. | | Metabolism | Etravirine is extensively metabolised by hepatic
microsomal enzymes, mainly by the cytochrome P450
isoenzymes CYP3A4, CYP2C9, and CYP2C19, to
substantially less active metabolites.Unchanged etravirine accounted for 81.2-86.4% of the
administered dose in faeces. Unchanged etravirine was
not detected in urine. |
| | Etravirine Preparation Products And Raw materials |
|