- Sodium Fusidate
-

- $0.00 / 1kg
-
2024-04-24
- CAS:751-94-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10000kg
- Sodium Fusidate
-

- $0.00 / 25kg
-
2024-04-12
- CAS:751-94-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000ton
- Sodium fusidate
-

- $0.00 / 1KG
-
2024-03-16
- CAS:751-94-0
- Min. Order: 100g
- Purity: 98%+
- Supply Ability: 100kg
|
| Product Name: | Sodium fusidate | | Synonyms: | fucidina;fusidatesodium;intertullefucidin;29-Nordammara-17(20),24-dien-21-oic acid, 16-(acetyloxy)-3,11-dihydroxy-, monosodium salt, (3.alpha.,4.alpha.,8.alpha.,9.beta.,11.alpha.,13.alpha.,14.beta.,16.beta.,17Z)-;FURSIDICACIDSODIUMSALT;29-Nor-8a,9b,13a,14b-dammara-17(20),24-dien-21-oic acid, 3a,11a,16b-trihydroxy-, 16-acetate, monosodium salt, (Z)- (8CI);29-Nordammara-17(20),24-dien-21-oic acid, 16-(acetyloxy)-3,11-dihydroxy-, monosodium salt, (3a,4a,8a,9b,11a,13a,14b,16b,17Z)- (9CI);ZN 6-Na | | CAS: | 751-94-0 | | MF: | C31H49NaO6 | | MW: | 540.72 | | EINECS: | 212-030-3 | | Product Categories: | antibiotic;Organics | | Mol File: | 751-94-0.mol | 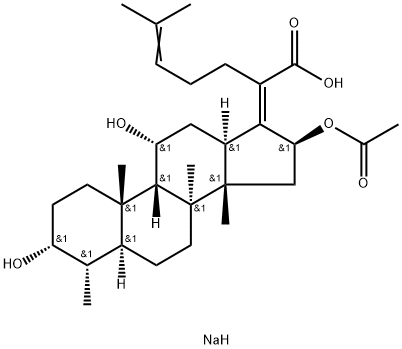 |
| | Sodium fusidate Chemical Properties |
| Melting point | >200°C (dec.) | | storage temp. | Keep in dark place,Inert atmosphere,2-8°C | | solubility | H2O: soluble | | color | White to Off-White | | Water Solubility | Soluble in water | | Sensitive | Moisture & Light Sensitive | | Merck | 13,4339 | | CAS DataBase Reference | 751-94-0(CAS DataBase Reference) |
| Hazard Codes | Xn,Xi | | Risk Statements | 22-36/37/38 | | Safety Statements | 26-36 | | WGK Germany | 3 | | RTECS | LV5775000 | | HS Code | 29419000 | | Toxicity | LD50 in mice (g/kg): 0.2 i.v. (Godtfredsen, Nature 1962) |
| | Sodium fusidate Usage And Synthesis |
| Narrow-spectrum antibiotics | Fusidic sodium mycophenolate sodium, also known as brown, fusidic sodium, having a steroid skeleton narrow-spectrum antibiotic, fusidic acid derivatives for the species, the appearance is white crystal or crystalline powder, odorless, bitter, slightly hygroscopic, soluble in water, ethanol, chloroform-soluble and insoluble in the acetone, ether, benzene. Danish LEO Pharma successfully developed the trade name Siding legislation for the first time, mainly in the liver metabolism, and by the excretion of bile, almost without going through the kidneys, low toxicity, have antiseptic effect by inhibiting bacterial protein synthesis. It has good antibacterial effect for gram-positive bacteria, it has good resistance. At the same time, this product is still sensitive for penicillin, methicillin antibiotic resistant strains such as, it has no cross resistance for husband sildenafil between sodium and other clinical use of antimicrobial agents.
This product is non cephalosporins. There is no need to do skin allergy test. The current clinical treatment has been widely used by a variety of sensitive bacteria, especially Staphylococcus aureus caused by various infections, such as osteomyelitis, septicemia, endocarditis , recurrent infections of cystic fibrosis, myocardial bacterial meningitis, pneumonia, meningitis, diphtheria, tuberculosis, skin and soft tissue infections, surgical wound infections and the like. Intravenous medication is used for severe infection or resistant strains infection, also used for MRSA infection. But due to its easy resistance, much as auxiliary drug use. Oral treatment failure caused by clostridium pseudo membranous enteritis has fine effect. | | Pharmacokinetics | Sodium fusidate(Fusidic acid) mainly works in the bacterial ribosome by inhibiting the displacement of the mRNA so as to block protein synthesis and sterilization. Good oral absorption, after a single oral dose of 0.5g 2h, Cmax about 14~38mg/L, 0.5g, 3 times/d, plasma concentration is up to 21~71mg/L in the 4th day. Drug-free savings, eating may affect the absorption. Intravenous infusion is 0.5g, 2h later, the plasma concentration is up to 40mg/L. High fat-soluble, tissue migration, and can penetrate into the aqueous humor, prostate, synovial fluid of the effective concentration; it can also smoothly go through the placenta, but it is difficult to penetrate the blood-brain barrier. Fusidic acid has excellent tissue penetration capability, which is widely distributed in the body. What is particularly important clinically is that this product is not only rich in high concentrations in the blood supply of tissues, It is known that the concentration of sodium fusidate exceeded the minimum inhibitory concentration for Staphylococcus (0.03 to 0.16 g/ml) in the pus, sputum, soft tissue, heart, bone tissue, synovial fluid, death of bone, burn eschar, brain abscess and intraocular. Fusidic sodium finishes its metabolism in the liver and is mainly excreted by the bile, hardly excreted by the kidneys. It has very low toxicity, and no cross allergy between other antimicrobial drugs in clinical use. Therefore it can be used to treat patients with contraindications to other antibiotics, such as penicillin or other antibiotics allergy. If serious and require prolonged treatment of deep infection. It is recommended that fusidic sodium associated with other anti-staphylococcal drugs can reduce drug resistance, It can obtain additive or synergistic effects result when associated with penicillin-resistant penicillins, cephalosporins, red vancomycin, aminoglycosides, clindamycin, rifampin or vancomycin. | | Adverse reactions and precautions | Adverse reactions were mild and slight, and there were slight gastrointestinal reactions in the oral administration. Intravenous injection of product can lead to thrombophlebitis and venous spasm. Daily use of 1.5 to 3 grams of reversible would increase aminotransferase according to some reports. There have been reports of individual patients after treatment may appear retrograde jaundice, which is mainly seen in the high-dose intravenous administration, especially in patients with severe S. aureus bacteremia. If persistent jaundice, you should stop using the drug to make the serum bilirubin return to normal. Allergic reactions were rarely reported.
Since the metabolism and excretion properties of fusidic sodium, when long-term large doses of medication or in combination with other fusidic sodium discharge pathway similar drugs (such as clindamycin or rifampicin), liver dysfunction and biliary abnormalities patients should regularly check liver function. In in vitro experiments, fusidic sodium may be substituted on the bilirubin albumin binding sites, such substitution clinical significance is unclear. It also did not discover the nuclear jaundices after using this product, but the premature children, jaundice, acidosis and severe sick newborns should pay attention to this factor when using this product. | | Application |
- Fusidic sodiumproduce bactericidal effectby inhibiting bacterial protein synthesis,and having strong antibacterial effecton a range for Gram-positive bacteria. Staphylococcus aureus, including penicillin, methicillin and other antibiotics-resistant strains are highly sensitive to the chemicals. It has no cross-resistance between fusidic sodium and other antibacterial drugs in clinical use.
- Role: steroid skeleton antibiotic mainly has effect against Gram-positive bacteria and Neisseria, Mycobacterium tuberculosis antibacterial. It is used for surrounding infection caused by susceptible strains, especially suitable for resistant to other antibiotics. Commonly used in the skin bone parts, joint infections etc. Packaging: 25 kg per cardboard barrel.
| | Description | Fusidic acid is a fusidane antibiotic originally isolated from F. coccineum. It is active against the Gram-positive bacteria S. aureus, S. pyogenes, C. diphtheriae, B. subtilis, and C. tetani (MIC50s = 0.01-20 μg/ml) but not the Gram-negative bacteria E. coli, S. typhimurium, and P. vulgaris or the fungi C. albicans and A. fumigatus (MIC50s = >100 μg/ml for all). Fusidic acid inhibits ribosomal recycling and protein translocation, processes mediated by elongation factor G (EF-G), in isolated E. coli ribosomes (IC50s = ~0.1 and ~200 μM, respectively). Topical administration of fusidic acid (2%) reduces the number of skin colony forming units (CFUs) and levels of TNF-α and IL-6 in a mouse model of methicillin-resistant S. aureus (MRSA) skin wound infection. | | Chemical Properties | White or almost white, crystalline powder, slightly hygroscopic. | | Uses | Antibacterial;Protein synthesis inhibitor GTPase coupled | | Uses | Sodium fusidate is the more water soluble sodium salt of fusidic acid, a steroidal metabolite of Fusidum coccineum which is a potent Gram positive antibiotic. Fusidic acid inhibits protein synthesis in prokaryotes by inhibiting the ribosome-dependent activity of G factor and translocation of peptidyl-tRNA. Fusidic acid also suppresses NO lysis of pancreatic islet cells. | | Uses | Sodium fusidate is the more water soluble sodium salt of fusidic acid, a steriodal metabolite of Fusidum coccineum which is a potent Gram positive antibiotic. Fusidic acid inhibits protein synthesis in prokaryotes by inhibiting the ribosome-dependent activity of G factor and translocation of peptidyl-tRNA. Fusidic acid also suppresses NO lysis of pancreatic islet cells. | | Brand name | Fucidine (Bristol-Myers Squibb). | | General Description | Fusidic acid is an antibiotic substance, resembling the structure of a steroid. It belongs to the class of fusidanes. | | Biochem/physiol Actions | Fusidic acid possesses antimicrobial action against skin pathogens. It is highly specific to Staphylococcus aureus. It is therefore used for treating infected traumatic wounds, folliculitis, furunculosis, impetigo, erythrasma and abscesses. | | Clinical Use | Antibacterial agent | | in vitro | resistance to fusidic acid in s. aureus strains was selected and mutations were identified in the fusa gene, which encodes ef-g. fusidic acid could not bind to free ef-g, but rather to ef-g gtp in the complex with ribosome, suggesting that the antibiotic required a specific conformation of ef-g for binding. biochemically, fusidic acid permited ribosome-stimulated gtp hydrolysis by ef-g, but prevented the associated conformational changes in ef-g, and therefore prevented ef-g turnover by stabilizing ef-g gdp on the ribosome. fusidic acid did not work on eukaryotes, but sordarin was considered to similarly act on yeast ef2 and was used to assemble ef2-80s yeast ribosome complexes for cryo-em analysis [1]. | | in vivo | animal study found that fusidic acid sodium salt was well absorbed after oral administration and it could significantly reduce the diabetes incidence in bb rats. fusidic acid sodium salt substantially accumulated more in female rats which might refult from the steroid structure of fusidin [2]. | | Drug interactions | Potentially hazardous interactions with other drugs
Antivirals: concentration of both drugs increased in
combination with ritonavir and saquinavir - avoid
with ritonavir.
Statins: increased risk of myopathy with simvastatin
and atorvastatin especially in diabetics. Avoid with
simvastatin and for 7 days after last dose.1 | | IC 50 | ~10-200 μm for the translocation function of the elongation factor ef-g | | Metabolism | Fusidic acid is excreted in the bile, almost entirely as
metabolites, some of which have weak antimicrobial
activity.
Approximately 2% appears unchanged in the faeces. | | references | [1] wilson, d. n. the a-z of bacterial translation inhibitors. crit. rev. biochem. mol. biol. 44(6), 393-433 (2009).
[2] hageman i, buschard k. antidiabetogenic effect of fusidic acid in diabetes prone bb rats: a sex-dependent organ accumulation of the drug is seen. pharmacol toxicol. 2002 sep;91(3):123-8. |
| | Sodium fusidate Preparation Products And Raw materials |
|