- CYTOCHALASIN B
-

- $1.00 / 1ASSAYS
-
2019-07-10
- CAS:14930-96-2
- Min. Order: 1ASSAYS
- Purity: 98%
- Supply Ability: 1kg,2kg,100kg
|
| | CYTOCHALASIN B Basic information |
| Product Name: | CYTOCHALASIN B | | Synonyms: | CytochalasinB from Drechslera dematioidea,Phomin;Cytochalasin B, froM Drechslera deMatoidea;Cytochalasin B (7S),(20R)-Dihydroxy-16(R)-methyl-10-phenyl-24-oxa[14]cytochalasa-6(12),13(E),21(E)-triene-1,23-dione;Cytochalasin B from Helminthosporium dematioideum
;ec[2,3-d]isoindole-2,18(5h)-dione;(E,E)-16-BENZYL-6,7,8,9,10,12A,13,14,15,15A,16,17-DODECAHYDRO-5,13-DIHYDROXY-9,15-DIMETHYL-14-METHYLENE-2H-OXACYCLOTETRADE[2,3-D]ISOINDOLE-2,18(5H)-DIONE;CYTOCHALASIN B, HELMINTHOSPORIUM DEMATIOIDEUM;CYTOCHALASIN B | | CAS: | 14930-96-2 | | MF: | C29H37NO5 | | MW: | 479.61 | | EINECS: | 239-000-2 | | Product Categories: | antibiotic | | Mol File: | 14930-96-2.mol | 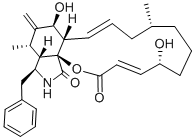 |
| | CYTOCHALASIN B Chemical Properties |
| Melting point | 218-223 °C | | Boiling point | 577.96°C (rough estimate) | | density | 1.1490 (rough estimate) | | refractive index | 1.6290 (estimate) | | Fp | 85℃ | | storage temp. | -20°C | | solubility | ethanol: 20 mg/mL | | form | powder | | pka | 13.60±0.70(Predicted) | | color | white | | Merck | 13,2819 | | BRN | 1096207 | | Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. | | InChIKey | UXVDBQLLXVKPRS-UZBFCAAUSA-N | | LogP | 3.370 | | EPA Substance Registry System | Cytochalasin B (14930-96-2) |
| | CYTOCHALASIN B Usage And Synthesis |
| Description | Cytochalasin B (14930-96-2) is a cell permeable fungal toxin which binds to the barbed end of actin, inhibiting its polymerization.1 Inhibits cell division, migration and glucose transport.2 Causes cell cycle arrest at G2/M and induces apoptosis in HCT-116 colorectal carcinoma cells.3? Cytochalasin B-induced membrane vesicles (CIMVs) retain cell surface receptors of the parent cells and retain fusion specificity with target cells.4 CIMVs are a promising new vector system for drug and biomolecule delivery due to their natural origin and participation in intercellular communication.5 | | Chemical Properties | white to off-white powder | | Uses | A cell-permeable fungal toxin used in actin polymerization studies and cytological | | Uses | Cytochalasin B is one of the most extensively studied members of a family of potent mycotoxins produced by several species of fungi. All members of the class exhibit profound effects on cytoskeletal proteins, giving rise to pronounced morphogenic activity in animals and plants. Like most cytochalsins, cytochalasin B exhibits potent inhibition of actin filament function leading to cell death by apoptosis, and displays a broad range of resultant cellular actions. Despite the common mode of action, there is evidence that individual members of this class display diverse selectivity. However, lack of comparative co-metabolite analysis has restricted a more complete understanding of their individual selectivity. | | Uses | As tools in cytological research and in characterization of polymerization properties of actin, q.v. | | Definition | ChEBI: An organic heterotricyclic compound, that is a mycotoxin which is cell permeable an an inhibitor of cytoplasmic division by blocking the formation of contractile microfilaments. | | General Description | Cytochalasin B is a cell-permeable fungal toxin / mycotoxin that binds to the ′barbed′ end of actin / actin filaments. This binding leads to:
- Disruption of actin filaments and of interaction of actin filaments in solution
- Inhibition of actin polymerization
- Inhibition of subunit association and dissociation
Cytochalasin B is widely used in studies of glucose transporters (GLUT). Cytochalasin B is also used as an integral part of various in vitro micronucleus assay protocols. | | Biochem/physiol Actions | Cell permeable fungal toxin that disrupts contractile microfilaments by inhibiting actin polymerization. This, in turn, induces DNA fragmentation, inhibits cell division, and disrupts many cell processes. Inhibits glucose transport. | | storage | Store at -20°C | | Purification Methods | Purify it by MeOH extraction, reverse phase C18 silica gel batch extraction by selective elution with 1:1 v/v hexane/tetrahydrofuran, crystallisation, subjected to TLC and recrystallisation [Lipski et al. Anal Biochem 161 332 1987]. It is soluble in EtOH (3.6%), Me2CO (1%), Me2SO (37%) and Me2NCHO (49%) at 24o, and can be crystallised from the first two solvents. It interferes with cellular movement [Korm Physiol Reviews 62 672 1982]. | | References | Threadoropoulos et al. (1994), Cytochalasin B may shorten actin filaments by a mechanism independent of barbed end capping; Biochem. Pharmacol., 47 1875
Whitesell et al. (2005), Compartmentalization of transport and phosphorylation of glucose in a hepatoma cell line; Biochem. J., 386 245
Buldak et al. (2014), Changes in subcellular localization of visfatin in human colorectal HCT-116 carcinoma cell line after cytochalasin B treatment; Eur. J. Histochem., 58 2408
Gomzikova et al. (2018), Evaluation of cytochalasin B-Induced Membrane Vesicles Fusion specificity with Target Cells; Biomed. Res. Int., 2018 7053623
Gomzikova et al. (2017), Cytochalasin B-induced membrane vesicles convey angiogenic activity of parental cells; Oncotarget, 8 70496 |
| | CYTOCHALASIN B Preparation Products And Raw materials |
|