|
|
| | 2-Amino-4,6-dichloro-pyrimidine-5-carbaldehyde Basic information |
| Product Name: | 2-Amino-4,6-dichloro-pyrimidine-5-carbaldehyde | | Synonyms: | 2-AMINO-4,6-DICHLORO-PYRIMIDINE-5-CARBALDEHYDE;2-AMINO-4,6-DICHLOROPYRIMIDINE-5-CARBOXALDEHYDE;2-AMINO-4,6-DICHLORO-5-FORMYL-PYRIMIDINE;2-AMINO-4,6-DICHLORO-5-PYRIMIDINECARBALDEHYDE;5-PYRIMIDINECARBOXALDEHYDE, 2-AMINO-4,6-DICHLORO-;2-amino-4,6-dichloro-5-pyrimid;2-AMINO-4,6-DICHLOROPYRIMIDINE-5-CARBOX&;2-azanyl-4,6-dichloro-pyrimidine-5-carbaldehyde | | CAS: | 5604-46-6 | | MF: | C5H3Cl2N3O | | MW: | 192 | | EINECS: | 624-720-5 | | Product Categories: | Heterocycle-Pyrimidine series;C1 to C6;C4 to C5;Carbonyl Compounds;Chemical Synthesis;Halogenated Heterocycles;Heterocyclic Building Blocks;Organic Building Blocks;Pyrimidines;Heterocycle intermediates;Pyridines, Pyrimidines, Purines and Pteredines;Aldehydes;Pyrazines, Pyrimidines & Pyridazines;Pyrimidine;Pyrazines, Pyrimidines & Pyridazines;Building Blocks;Halogenated Heterocycles;Heterocyclic Building Blocks;PyrimidinesHeterocyclic Building Blocks;Building Blocks | | Mol File: | 5604-46-6.mol | 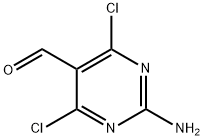 |
| | 2-Amino-4,6-dichloro-pyrimidine-5-carbaldehyde Chemical Properties |
| Melting point | 208-224 °C | | Boiling point | 411.6±55.0 °C(Predicted) | | density | 1.688±0.06 g/cm3(Predicted) | | storage temp. | 2-8°C | | form | powder | | pka | -1.57±0.10(Predicted) | | color | Pale Yellow | | Sensitive | Air Sensitive | | CAS DataBase Reference | 5604-46-6(CAS DataBase Reference) |
| | 2-Amino-4,6-dichloro-pyrimidine-5-carbaldehyde Usage And Synthesis |
| Chemical Properties | White solid | | Uses | Substrate used in the synthesis of an N-terminal surrogate in amino acid and peptide analogues. | | Uses | 2-Amino-4,6-dichloro-pyrimidine-5-carbaldehyde is a substrate used in the synthesis of an N-terminal surrogate in amino acid and peptide analogues. | | Synthesis | Example 1: This example describes the synthesis of 2-amino-4,6-dichloropyrimidine-5-carbaldehyde using 2-amino-4,6-dihydroxypyrimidine as starting material. The procedure was as follows: absolute DMF (210 mL, 1.38 mol) was added dropwise to an ice-cold POCl3 solution (900 mL) over 20 minutes under ice bath conditions. After removal of the ice bath, powdered 2-amino-6-hydroxypyrimidin-4(3H)-one (150 g, 1.17 mol) was added over 20 min. Subsequently, the reaction mixture was heated to 100 °C and the reaction was stirred at this temperature for 3-4 hours. Upon completion of the reaction, the solution was cooled to room temperature and slowly poured into a pre-prepared ice-water mixture (diluted to 10 L from 4 L of crushed ice with water). The resulting aqueous solution was heated to 50 °C and stirring was continued for 2 hours. Finally, the mixture was placed in a refrigerator overnight and the precipitate was collected by filtration and dried to obtain the target product 2-amino-4,6-dichloropyrimidine-5-carbaldehyde (160 g, 71% yield). | | References | [1] Patent: US2003/78413, 2003, A1 |
| | 2-Amino-4,6-dichloro-pyrimidine-5-carbaldehyde Preparation Products And Raw materials |
|