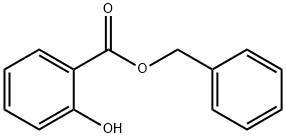
Synthesis and Sensitization Study of Benzyl Salicylate
Dec 12,2025
Benzyl Salicylate is a food flavor permitted by GB2760-1996, primarily used in formulating apricot, peach, banana, and raspberry flavor profiles. As a fragrance ingredient, it serves as a solvent for nitro musks, a fixative for artificial musks and floral scents, and a preservative with antibacterial properties. This versatile Benzyl Salicylate is widely employed as both a co-solvent and effective fixative in floral and non-floral perfumes—such as carnation, ylang-ylang, jasmine, violet, lily-of-the-valley, lilac, and tuberose—and can also be used in trace amounts in edible flavors including apricot, peach, plum, banana, and pear.

Figure1: Picture of Benzyl salicylate
General Introduction
Benzyl salicylate (benzyl-o-hydroxybenzoate: CAS 118-58-1) is an important raw material used in many different fragrances for its sweet, balsamic odour and solvent properties. It has been in public use since the 1920s and the soap and detergent industry has been a major user. An estimated 750,000 lb is used annu- ally in perfumes and flavourings in the USA. Benzyl salicylate is made by reacting sodium salicylate with benzyl chloride and is > 98% pure (EOA, 1975). It proved for food use by the Food and Drug Adminis- tration (21 CFR 121.1164). Commercial benzyl salicylate is made by reacting sodium salicylate with benzyl chloride and is > 98~/O pure (EOA, 1975). It also occurs in numerous natural oils including ylang ylang oil and carnation oil.
Synthesis
Synthesis of benzyl salicylate from sodium salicylate and benzyl chloride in the absence of a PTC and with dimethyl formamide as a solvent has been reported. Almost complete conversion of benzyl chloride can be achieved in 1.5 h at 110 °C. The batch time and the reaction temperature are considerably less than that for the commercial process using a PTC. Kinetics of the reaction have been investigated. [1]
Sensitization Study
Benzyl salicylate has been documented in clinical reports as a potential sensitizer in dermatitic patients, with studies by Hjorth (1961) linking it to Peru balsam hypersensitivity, Kahn (1971) observing contact sensitization in 6 of 15 patients after 8-MOP phototherapy, Mitchell (1975) reporting 38 sensitized cases among 183 Japanese dermatitis patients, Larsen (1977) detecting reactions to 2% benzyl salicylate in 2 of 20 patients, and Rudner (1977) noting a 2.5% response rate (183 subjects). Contrasting with these findings in dermatitic individuals, the study systematically evaluates both pre-existing sensitization to this compound and its potential to induce delayed hypersensitivity in non-dermatitic subjects through testing with consumer products and fragrance blends containing the material, presenting previously unpublished data. The potential of benzyl salicylate, an important fragrance and flavour ingredient, to induce hypersensitivity or to elicit reactions to pre-existing hypersensitivity in the general population was evaluated by analysing patch-test data. Results obtained from fragrance and formulator companies for a total of 10,538 patch tests on benzyl salicylate alone, on a variety of household and personal care consumer products and on fragrance blends containing this compound were analysed as part of this survey. No induced or elicited responses directly attributable to benzyl salicylate were observed in the 35 patch tests on benzyl salicylate alone, or in the 10,503 patch tests on consumer products or fragrance blends containing benzyl salicylate. The highest concentration of this compound tested in the consumer-product tests was 2 × 101%, and this compound alone was tested at 10% in ethanol. This study indicates that benzyl salicylate has a very low potential to induce hypersensitivity ('induced' reactions) or to elicit reactions presumably attributable to pre-existing sensitization ('elicited' reactions) and thus supports the safe use of benzyl salicylate in consumer products and fragrance blends. [2]
Safety Issues
The findings of investigations demonstrate that benzyl salicylate is unlikely to induce hypersensitivity or elicit reactions in pre-sensitized individuals under real-world exposure scenarios through consumer products containing this fragrance ingredient. With a substantially expanded dataset compared to previous studies, these results strongly support the continued safe use of benzyl salicylate in commercial consumer formulations. [2]
Reference
[1] Sivakumar S , Pangarkar V G , Sawant S B .Homogeneous System for the Synthesis of Benzyl Salicylate[J].Organic Process Research & Development, 2002, 6:149-151.
[2] Kohrman K A , Booman K A , Dorsky J ,et al.Benzyl salicylate: a survey of consumer patch-test sensitization[J]. Food & Chemical Toxicology, 1983, 21:741-744.
- Related articles
- Related Qustion
- Is Benzyl Salicylate the same as salicylic acid? Nov 29, 2024
Benzyl salicylate is a derivative of salicylic acid, but it is different from salicylic acid.
Diosgenin is a precursor of many steroidal drugs synthesis, an important raw material for artificial synthesis of steroid hormones and steroidal contraceptives.....
Dec 12,2025Biochemical EngineeringBenzyl salicylate
118-58-1You may like
Benzyl salicylate manufacturers
- Benzyl salicylate
-

- $0.00 / 25KG
- 2025-12-12
- CAS:118-58-1
- Min. Order: 25KG
- Purity: 99%min
- Supply Ability: 30tons/month
- Benzyl salicylate
-
- $1.00 / 1KG
- 2025-12-11
- CAS:118-58-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- Benzyl salicylate
-
- $0.00 / 1removed
- 2025-12-05
- CAS:118-58-1
- Min. Order:
- Purity:
- Supply Ability: 10g