Synthesis and Synthetic Applications of 1,4-Dioxaspiro[4.5]decan-8-one
Dec 10,2025
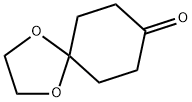
1,4-Dioxaspiro[4.5]decan-8-one, a ketal-type fundamental building block in organic synthesis, appears as a white to off-white solid powder under ambient conditions and is highly acid-labile, readily undergoing hydrolytic degradation in the presence of acidic substances to yield the corresponding diketone derivatives. As a primary chemical intermediate in pharmaceutical chemistry, 1,4-Dioxaspiro[4.5]decan-8-one has been reported to serve as a key construct for synthesizing tritium-labeled probes, which can be utilized in radioligand binding assays for the study of dopamine reuptake complexes.
Synthesis
![Synthesis of 1,4-Dioxaspiro[4.5]decan-8-one Synthesis of 1,4-Dioxaspiro[4.5]decan-8-one](/NewsImg/2025-11-26/6389979647696608952235715.jpg)
Figure1 : Synthesis of 1,4-Dioxaspiro[4.5]decan-8-one
Method 1
A mixture of 1,4-cyclohexanedione (5.00 g, 45.6 mmol, 1.00 eq.), p-toluenesulfonic acid (0.848 g, 4.56 mmol, 0.10 eq.), and ethylene glycol (3.32 g, 53.5 mmol, 1.2 eq.) was dissolved in toluene (45.0 mL) in a round-bottom flask equipped with a Dean-Stark apparatus, heated to 120°C until 1.2 mL of water was collected azeotropically, and then quenched with saturated sodium bicarbonate solution; the aqueous layer was extracted with ethyl acetate (2 x 30 mL), and the combined organic layer was subsequently washed with saturated sodium bicarbonate solution (2 x 30 mL), water (2 x 30 mL), and brine (1 x 30 mL), dried over sodium sulfate, decanted, and concentrated to dryness, with final purification by flash chromatography on silica gel using 50% ethyl acetate in hexane (Rf = 0.5) to afford the cyclohexadiene ditriflate monomer 1,4-dioxaspiro[4.5]decan-8-one. [1]
Method 2
At ambient temperature, concentrated sulfuric acid (1.5–3.0 g) was added to the reaction solution, and the mixture was stirred for 10 hours; the resulting reaction mixture was then washed with saturated aqueous sodium bicarbonate solution (100 mL), after which the organic layer was separated from the resulting biphasic mixture, and the aqueous layer was further extracted with DCM (3 × 100 mL). The combined organic layers were dried over sodium sulfate and concentrated by rotary evaporation to afford the crude product 1,4-dioxaspiro[4.5]decan-8-one. [2]
Synthetic Applications
Synthesis of Spiroquinazolinamine Derivatives
A series of 6-amino-8-aryl-3,4-dihydro-1H-spiro[naphthalene-2,2'-[1,3]dioxolane]-5,7-dicarbonitrile and 8-arylidene-4-aryl-7,8-dihydro-5H-spiro[quinazoline-6,2'-[1,3]dioxolan]-2-amine derivatives were synthesized in high yields through reactions of 1,4-dioxaspiro[4.5]decan-8-one with aromatic aldehydes and malononitrile (or guanidine carbonate) under different conditions, where the DBU-THF system proved efficient for producing the former spiro naphthalene derivatives while 95% EtOH with NaOH was preferred for obtaining the latter spiro quinazoline derivatives—marking the first reported synthesis of these spiroheterocycles—with additional advantages including short reaction times, broad substrate scope, and simple experimental setup.
Total Synthesis of Bifidenone
The research team accomplished the first total synthesis of the novel natural tubulin polymerization inhibitor bifidenone through a 12-step sequence starting from commercially available 1,4-dioxaspiro[4.5]decan-8-one. Their approach features a newly developed palladium-catalyzed aerobic dehydrogenation to construct the dihydrobenzodioxolone core and establishes the three stereocenters via an AD-mix-β dihydroxylation, followed by a late-stage palladium-catalyzed decarboxylation–allylation. Using 1,4-dioxaspiro[4.5]decan-8-one as the molecular scaffold, the team ultimately determined the absolute stereochemistry of compound 3 by single-crystal X-ray analysis of intermediate. [3]
Total Synthesis of (+)-α-lycorane
The research team achieved the synthesis of optically active (+)-α-lycorane in 19.6% overall yield through a 13-step sequence starting from commercially available 1,4-dioxaspiro[4.5]decan-8-one, employing a ruthenium-catalyzed asymmetric hydrogenation as the key step. The first step of the experiment:A stirred solution of bromobenzene (20 mmol) in anhydrous THF (50 mL) under a nitrogen atmosphere was cooled to –40 °C and treated dropwise with n-butyllithium (2.5 M in hexane, 8 mL, 20 mmol). After stirring at –40 °C for 30 minutes, the mixture was treated with a solution of 1,4-dioxaspiro[4.5]decan-8-one (3.1 g, 20 mmol) in THF (20 mL), then allowed to warm to room temperature and stirred for an additional hour before being quenched with saturated aqueous NH₄Cl (20 mL). The layers were separated, and the aqueous layer was extracted with ethyl acetate (2 × 40 mL); the combined organic extracts were then dried over MgSO₄ and concentrated in vacuo to afford 8-aryl-1,4-dioxaspiro[4.5]decan-8-ol, which was used directly in the subsequent step without further purification. [4]
Reference
[1] Pomfret, Meredith N.; et al, Stochastic Bullvalene Architecture Modulates Structural Rigidity in π-Rich Macromolecules, Angewandte Chemie, International Edition 2023, 62, e202301695.
[2] Tu, Yi-Min; et al, Insights into substitution strategy towards thermodynamic and property regulation of chemically recyclable polymers, Nature Communications 2023, 14, 3198.
[3] Huang Z , Williams R B ,O’Neil-Johnson, Mark,et al.A Total Synthesis of Bifidenone[J].Journal of Organic Chemistry, 2017, 82:4235-4241.
[4] Li G, Xie J H, Hou J, et al. Catalytic Asymmetric Hydrogenation of α‐Arylcyclohexanones and Total Synthesis of (?)-Lycorane[J]. Advanced Synthesis & Catalysis, 2013, 355: 1597-1604.
- Related articles
- Related Qustion
Ribociclib (Kisqali) is a targeted therapy approved by the Food and Drug Administration to treat stage II, stage III, and advanced or metastatic hormone receptor-positive, HER2-negative breast cancer.....
Dec 10,2025Biochemical EngineeringRibociclib is used in combination with another medication to treat a certain type of hormone receptor–positive (depends on hormones such as estrogen to grow) advanced breast cancer.....
Dec 10,2025Biochemical Engineering1,4-Dioxaspiro[4.5]decan-8-one
4746-97-8You may like
1,4-Dioxaspiro[4.5]decan-8-one manufacturers
- 1,4-Dioxaspiro[4.5]decan-8-one
-
![4746-97-8 1,4-Dioxaspiro[4.5]decan-8-one](/ProductImageEN/2021-11/Small/de52e029-fe88-431b-861e-6c2db3aae4d1.jpg)
- $10.00 / 1KG
- 2025-12-11
- CAS:4746-97-8
- Min. Order: 100KG
- Purity: 99%
- Supply Ability: 100 mt
- 1,4-Cyclohexanedione monoethyleneacetal
-

- $20.00 / 1KG
- 2025-12-11
- CAS:4746-97-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 300 Tons/ year
- 1,4-Dioxaspiro[4.5]decan-8-one
-
![4746-97-8 1,4-Dioxaspiro[4.5]decan-8-one](/ProductImageEN/2023-01/Small/eaf66483-93e1-452d-8f0b-275158ebe93e.jpg)
- $0.00 / 1KG
- 2025-12-11
- CAS:4746-97-8
- Min. Order: 1KG
- Purity: 98%min
- Supply Ability: 30tons/month