17-Methyltestosterone: synthesis and chemical reactions
Jul 3,2023
![]()
![]()
![]()
![]()
![]() General Description
General Description
17-Methyltestosterone is a synthetic derivative of testosterone used in healthcare steroid manufacturing. It is made from natural products like diosgenin. The synthesis involves converting diosgenin to 33-hydroxyandrost-5-en-17-one through chemical reactions. This compound is then treated with methylmagnesium iodide to produce 17-methyltestosterone. Further chemical reactions modify 17-methyltestosterone to create different derivatives with unique properties. These advancements have contributed to the steroid industry and the production of compounds with various applications.

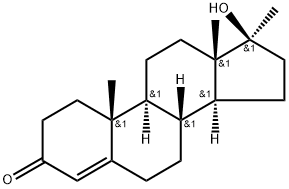
Figure 1. 17-Methyltestosterone
Synthesis
Chemists arrive now at the threshold of health care steroid manufacture affected by biotechnology. In relation to market rate, steroid manufacturing as well as antibiotic production is of the highest priority. Diosgenin and solasodine are natural products used in hormone industry as starting materials. In 1938, Marker achieved the first practical synthesis of the pregnancy hormone, progesterone, and 3β-hydroxypregn-5-en-20-one from diosgenin that is known as “Marker Degradation”. 3β-Hydroxypregn-5-en-20-one produces 3β-hydroxyandrost-5-en-17-one which is the precursor for the synthesis of 17α-methyltestosterone hormone. Oxygenation of a solution of 3β-hydroxypregn-5-en-20-one in a mixture of tetrahydrofuran and tert-butanol containing potassium tert-butoxide at 5–7°C gave pregnenolone hydroperoxide when 1.1 M equivalents of oxygen have been consumed. Nitrogen is passed through the solution for several minutes and the reaction mixture is then heated to 60–70°C to provide 3β-hydroxyandrost-5-en-17-one which on treatment with methylmagnesium iodide afforded 17α-methyl compound. Oppenauer oxidation of 17α-methyl compound formed directly 17 β-hydroxy-17α-methylandrost-4-en-3-one. Solvent loss technique was then employed for the growth of transparent plate shaped crystals using methanol as the solvent system. 1
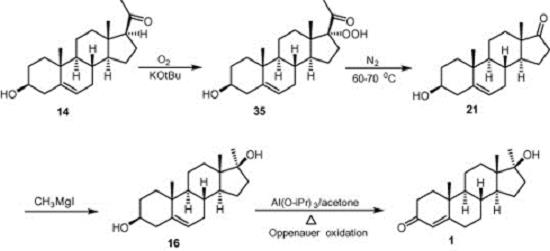
Figure 2. Synthesis of 17-methyltestosterone
Chemical reactions
![]() Alkylation reaction
Alkylation reaction
The alkylation reactions of 17-methyltestosterone can yield various products depending on the alkylating reagents and reaction conditions used. In one scenario, when reacted with methyl iodide in the presence of potassium tert-butoxide, tert-butyl alcohol as a solvent, and at specific reaction temperatures, it leads to the formation of 17β-hydroxy-4,17α-dimethyandrost-4-en-3-one and 17β-hydroxy-4,4,17α-trimethylandrost-5-en-3-one. The reaction temperature plays a role in determining the yield of these products. Another alkylation reaction involves treating 17α-methyltestosterone with ethyl bromide under the same basic conditions at room temperature for 16 hours. This results in the formation of 4-ethyl-17β-hydroxy-17α-methylandrost-4-en-3-one and 4,4-diethyl-17β-hydroxy-17α-methylandrost-5-en-3-one with yields of 20% and 60%, respectively. Furthermore, by refluxing a mixture of 17-methyltestosterone, thiophenol, 38% formaldehyde, and triethylamine in ethanol for 55 hours, 17β-hydroxy-17α-methyl-4-(phenylthiomethyl)androst-4-en-3-one can be obtained with a yield of 75%. Subsequent desulfurization of compound 42 using catalytic Raney nickel-water sludge through gentle refluxing for 5 hours gives 82% yield of 17β-hydroxy-4,17α-dimethylandrost-4-en-3-one. These alkylation reactions demonstrate the ability to introduce alkyl groups to specific positions on the 17-methyltestosterone molecule, leading to the formation of various derivatives with modified structures and properties. 2
![]()
![]() Condensation reaction
Condensation reaction
17-Methyltestosterone can undergo condensation reactions with various reagents to form different derivatives. When reacted with semicarbazide, it forms the corresponding semicarbazone derivative. Similarly, heating 1 with isonicotinic acid hydrazide in absolute ethanol containing 2.5% ethanolic HCl results in the formation of the isonicotinohydrazone derivative. Heating 17-methyltestosterone with dimethylhydrazine and a few drops of acetic acid leads to the synthesis of the corresponding steroidal hydrazone derivative with a yield of 96%. Additionally, condensation of 17-methyltestosterone with 2,4-dinitrophenylhydrazine gives rise to the steroidal arylhydrazone derivative. Condensing 17 -methyltestosterone with phthalazinehydrazide produces the phthalazinohydrazone derivative with a yield of 86%. These condensation reactions provide methods to modify the structure of 17-methyltestosterone by introducing functional groups such as semicarbazone, isonicotinohydrazone, steroidal hydrazone, arylhydrazone, and phthalazinohydrazone, leading to the formation of novel compounds with potential applications. 3
Wittig reaction
The keto group in 17-methyltestosterone, can be removed through a process called the Wittig reaction. In this reaction, methylenetriphenylphosphorane is used as the reagent. Methylenetriphenylphosphorane is prepared beforehand by reacting methyltriphenylphosphonium bromide with a 1.3 N phenyllithium solution in dry ether. When 17-methyltestosterone is treated with methylenetriphenylphosphorane, it undergoes the Wittig reaction, resulting in the formation of 3-methylene-17α-methylandrost-4-en-17β-ol. The yield of this product is reported to be 45.25% based on the starting material. In summary, the Wittig reaction using methylenetriphenylphosphorane is employed to remove the keto group from 17-methyltestosterone, leading to the synthesis of 3-methylene-17α-methylandrost-4-en-17β-ol. 3
Reference
1. Verma R, Jasrotia D, Bhat M. Synthesis and
X-ray crystallographic analysis of 17α-hydroxy-17-
methylandrost-4-ene-17-one. Journal of Chemical Crystallography, 2006,
36(5):283-287.
2. Manson AJ, Stonner FW, Neumann HC, et al. Steroidal heterocycles. VII. Androstano(2,3-D)isoxazoles and related compounds. J Med Chem, 1963, 6:1-9.
3. El-Desoky el-SI, Reyad M, Afsah EM, Dawidar AA. Synthesis and chemical reactions of the steroidal hormone 17α-methyltestosterone. Steroids, 2016, 105:68-95.
- Related articles
- Related Qustion
- 17-Methyltestosterone: Overview and Effects in Pseudorasbora Parva Feb 7, 2024
17-Methyltestosterone, an endocrine-disrupting compound, disrupts fish reproduction and contaminates water, prompting bans and highlighting the need for further environmental research.
- What is 17-Methyltestosterone? Sep 14, 2021
Methyltestosterone (17-Methyltestosterone), whose chemical name is 17b-hydroxy-17a-methylandrost-4-en-3-one, is a hormone steroid drug developed by the former Ciba company in Switzerland and has been marketed in many countries.
17-Methyltestosterone
58-18-4You may like
17-Methyltestosterone manufacturers
- Methyltestosterone
-

- $1.00 / 1KG
- 2025-12-17
- CAS:58-18-4
- Min. Order: 1KG
- Purity: 99.9%
- Supply Ability: 500KG
- Methyl testosterone
-

- $0.00 / 1kg
- 2025-12-16
- CAS:58-18-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000kg
- Methyltestosterone
-

- $100.00 / 1Box
- 2025-12-14
- CAS:58-18-4
- Min. Order: 1Box
- Purity: 99.99%
- Supply Ability: 20000 boxes