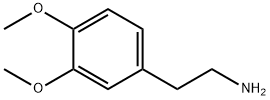
3,4-Dimethoxyphenethylamine: A Phenethylamine Analogue
Dec 18,2025
3,4-Dimethoxyphenethylamine (DMPEA) is a chemical compound of the phenethylamine class. It is an analogue of dopamine where the 3- and 4-position hydroxy groups have been replaced with methoxy groups. It is also closely related to mescaline which is 3,4,5-trimethoxyphenethylamine. DMPEA was first synthesized and assayed by Alexander Shulgin. In his book PiHKAL, he describes 3,4-Dimethoxyphenethylamine as producing no effects when tested even with very high doses, such as 1,000 mg orally or 10 mg via intravenous injection. As a result, it can be assumed that it is biologically inactive. However, it has been shown to have some activity as a monoamine oxidase inhibitor.

3,4-Dimethoxyphenethylamine in the US military's search for a truth drug
This article provides an outline of the history about how some “mescaline derivatives” such as 3,4,5-trimethoxyamphetamine (TMA), 3,4-methylenedioxyamphetamine (MDA), 3,4-methylenedioxy-N-ethylamphetamine (MDE) and 3,4-methylenedioxymethamphetamine (MDMA) were synthesized and experimented with by the military. These mescaline derivatives like 3,4-Dimethoxyphenethylamine were not researched as psychochemicals for mass intoxications in “unconventional warfare” as was lysergic acid diethylamide (LSD). In the 1920s, American neurologist Heinrich Klüver and pharmacologist Gordon A. Alles were interested in mescaline. Klüver's research on mescaline focused on the “mechanisms of hallucination.” He noted that during the mescaline inebriation that “the ability to organize and to abstract material is lost; the determining tendencies suffer. To concentrate on something for a long while becomes impossible.” During the 1930s, Klüver evaluated the synthetic mescaline derivatives 3,4-dimethoxyamphetamine (DMA), 3,4-dimethoxyphenethylamine (DMPEA), amphetamine and MDA in animals. All four substances subsequently became part of military research. After insufficient animal testing, the substances were given to patients at the New York State Psychiatric Institute (NYSPI). 3,4-Methylenedioxy-N-ethylamphetamine (MDE), a compound almost identical to MDMA, was among the compounds delivered for testing at the NYSPI. During tests with other derivatives (3,4-dimethoxyphenethylamine) 3,4-methylenedioxyphenethylamine (MDPEA), MDA) in 1952–53, an unwitting patient died in these tests, which was kept secret from the public. [1]
Synthesis of novel sulfonamides with anti-Alzheimer
β-Phenylethylamine (βPEA) is a trace amine that is deemed to have an important role in the central nervous system of animals. However, its effects on the brain are still not completely understood. Phenylethylamine derivatives are compounds that form a large class of both biochemicals and organic synthetic compounds. Dopamine, norepinephrine, and serotonin are biochemical derivatives and neurotransmitters that regulate many body functions. Phenylethylamine and substituted derivatives are widely used as active drug substances. Therefore, their synthesis and the investigation of their biological activities have been under consideration for the development of new drugs. Dimethylcarbamoyl chloride is used in the synthesis of biologically active carbamates. Dimethyl urea was obtained from the reaction of dimethylcarbamoyl chloride and 3,4-dimethoxyphenethylamine in the presence of NEt3. HSO3Cl is a strong acid and conventionally used in chlorosulfonylation reactions of aromatic compounds. 3,4-dimethoxyphenethylamine was converted into urea and novel sulfonamides . All synthesized compounds were investigated for their inhibitory effect on AChE and BChE enzymes and antioxidant capacities.[2]
A series of novel dopamine analogs incorporating urea and sulfonamide functional groups was synthesized from 3,4-dimethoxyphenethylamine. For the DPPH• radical scavenging method, compounds have a much better scavenging power than the standard molecules. In addition, it has been determined by the induced-fit docking method that compound 13 is well-fitted in the active site of the enzymes. ADME studies reveal that the pharmacokinetic and physicochemical properties of all synthesized compounds are within an acceptable range. The reaction of 3,4-dimethoxyphenethylamine with N,N-dimethylcarbamoyl chloride, followed by the sulfonyl chlorination of the urea derivative, gave benzene-1-sulfonyl chloride, which was reacted with NH3 (aq) or N-alkyl amines to give related sulfonamides. The O-demethylation reaction of the subsequent compounds with BBr3 afforded four novel phenolic dopamine analogs including sulfonamide and urea in the same structure. The anticholinergic and antioxidant effects of the synthesized compounds were examined.
References
[1]Passie, Torsten, and Udo Benzenhöfer. “MDA, MDMA, and other "mescaline-like" substances in the US military's search for a truth drug (1940s to 1960s).” Drug testing and analysis vol. 10,1 (2018): 72-80. doi:10.1002/dta.2292
[2]Gök, Nihal et al. “Synthesis of novel sulfonamides with anti-Alzheimer and antioxidant capacities.” Archiv der Pharmazie vol. 354,7 (2021): e2000496. doi:10.1002/ardp.202000496
- Related articles
- Related Qustion
Ethylene glycol diglycidyl ether (aliphatic epoxy resin) acts as epoxy diluent and chitosan crosslinker, enabling ultrasound-responsive drug release.....
Dec 18,2025Organic SolventsNandrolone, an anabolic-androgenic steroid, has been prohibited by doping control regulations for more than 30 years. Let us learn about it.....
Dec 18,2025Drugs3,4-Dimethoxyphenethylamine
120-20-7You may like
3,4-Dimethoxyphenethylamine manufacturers
- 3,4-Dimethoxyphenethylamine
-
- 2025-12-18
- CAS:120-20-7
- Min. Order:
- Purity: 0.99
- Supply Ability:
- 3,4-Dimethoxyphenethylamine
-
- $50.00 / 500mg
- 2025-12-18
- CAS:120-20-7
- Min. Order:
- Purity: 99.50%
- Supply Ability: 10g
- 3,4-Dimethoxyphenylethylamine
-

- $20.00 / 1KG
- 2025-12-18
- CAS:120-20-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 300 Tons/ year