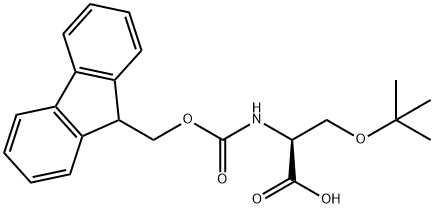
FMOC-O-tert-butyl-L-serine: Synthesis & Biological Application
Dec 18,2025
FMOC-O-tert-butyl-L-serine is an important protecting group amino acid. As a key intermediate in peptide synthesis, it possesses unique application value due to the dual protective groups within its structure. Its solubility exhibits distinctive behaviour: insoluble in water and petroleum ether, yet readily soluble in organic solvents such as chloroform, dichloromethane, ethyl acetate, and DMSO. Preparation methods of FMOC-O-tert-Butyl-L-serine comprise laboratory and industrial pathways: Laboratory synthesis typically involves reacting O-tert-butyl-L-serine with furanmethoxycarbonyl azide, followed by pH-controlled extraction and purification. Industrial production involves saponification of L-serine methyl ester hydrochloride with isobutylene, followed by acylation and acidification extraction, yielding products with over 99% purity. This material is also utilised in preparing low-molecular-weight gelling agents and functions in scenarios like dye removal. Storage requires sealing at 2–8°C, away from oxidising agents. Handling necessitates protective measures to prevent dust inhalation.

Synthesis of Cetrorelix Acetate by Using Fmoc Solid-Phase Peptide
In the synthesis of cetrorelix via solid-phase peptide synthesis (SPPS) employing the Fmoc strategy, the racemization of L-arginine and L-serine was effectively minimized to below 0.5%. This reduction was achieved using the coupling agent HATU, the additive HOBt or HOAt, and the base TMP. Racemization was observed during the coupling of Fmoc-L-arginine(Pbf) and Fmoc-O-tert-butyl-L-serine on Rink Amide AM resin. A gradient reversed-phase high-performance liquid chromatography (RP-HPLC) method was developed for the separation of all the structurally closely related cetrorelix isomers. Optimized RP-HPLC conditions identified D-arginine and D-serine isomeric impurities as the closest eluting peaks to the main cetrorelix peak. Controlling these impurities to the lowest possible levels is essential for developing an efficient preparative HPLC purification process for the commercial production of cetrorelix. This stringent control ensures that the final product meets the high standards required for commercial production and therapeutic use with FMOC-O-tert-Butyl-L-serine.[1]
Preparation of FMOC-O-tert-Butyl-L-serine
In the present example, labeled FMOC-O-tert-Butyl-L-serine was prepared. Specifically, 1.260 g of Fmoc-L-(O-tert-butyl)serine N-hydroxysuccinimide ester (Novabiochem (Darmstadt, Germany)) and 450 mg of anhydrous HOBt were dissolved in 4.0 mL of anhydrous THF. Immediately after then, 200 µL of H2 17 0-enriched water (labeling rate 20.3 %) was added, and the mixture was stirred at room temperature for 4 to 5 days. After the reaction has been completed, the reaction mixture was evaporated to dryness under reduced pressure to give a white product. The crude product was purified by chromatography on silica gel (100:2(v/v) ethyl acetate : acetic acid) to give labeled FMOC-O-tert-Butyl-L-serine.[2]
2,3-Diaminopropanols Obtained from Serine as Intermediates
2,3-l-Diaminopropanoic acid (l-Dap) is a non-proteinogenic amino acid produced by numerous plants and bacteria. It is the main structural motif of albizziine, a natural non-proteinaceous free amino acid which is ubiquitously present in higher plants and in seeds. The last decade was characterized by the disclosure of a wide range of synthetic routes to l-Dap and its analogues. Much effort was spent searching for convenient methods which could employ simple molecular scaffolds. Natural amino acids were proposed as convenient starting materials; however, the use of serine was seldom investigated. In particular, our synthetic plan was chalked out starting from optically pure protected d-serine. The key synthetic step of the procedure was the Ti(OiPr)4-assisted reductive amination of a protected FMOC-O-tert-Butyl-L-serine derivative to give a series of protected 2,3-diaminopropanols, which were oxidized under mild conditions. It is worth noting that FMOC-O-tert-Butyl-L-serine was used to obtain the correct l-Dap stereochemistry.[3]
In summary, a synthetic route is proposed for the multi-step preparation of orthogonal di-protected l-Dap methyl esters. In the proposed procedure, commercial FMOC-O-tert-Butyl-L-serine is initially transformed into the corresponding serinal. This compound is subjected to a Ti(IV)-assisted reductive amination with amines and arylsulfonamides, to give 2,3-diaminopropanols which are in turn oxidized by the TCCA/TEMPO system. The obtained carboxylic acids are finally methylated, to yield the targeted esters in 68%–73% overall yields. Reductive amination allows also the preparation of N-methylated and N-ethylated 2,3-diaminopropanol intermediates as the most likely precursors of N-alkylated l-Dap scaffolds. Chromatographic purifications are minimized through the entire procedure. The total preservation of chirality enhanced the synthetic utility of the method, which can successfully be used to prepare protected FMOC-O-tert-Butyl-L-serine building blocks for a broad application in the synthesis of biologically active peptides.
References
[1]Aluri, Kiran et al. “Synthesis of Diastereomerically Pure Cetrorelix Acetate by Using Fmoc Solid-Phase Peptide Synthesis (SPPS) Strategy: A Commercially Viable Approach.” Journal of peptide science : an official publication of the European Peptide Society vol. 31,6 (2025): e70030. doi:10.1002/psc.70030
[2]INSTITUTE OF PHYSICAL AND CHEMICAL RESEARCH RIKEN - EP1757568, 2007, A1
[3]Temperini A, Aiello D, Mazzotti F, Athanassopoulos CM, De Luca P, Siciliano C. 2,3-Diaminopropanols Obtained from d-Serine as Intermediates in the Synthesis of Protected 2,3-l-Diaminopropanoic Acid (l-Dap) Methyl Esters. Molecules. 2020 Mar 13;25(6):1313. doi: 10.3390/molecules25061313. PMID: 32183079; PMCID: PMC7145313.
- Related articles
- Related Qustion
Glucose is a fundamental substance sustaining human life activities. Its synthesis pathways differ across organisms. In plants, glucose is synthesized through photosynthesis within chloroplasts of plant cells.....
Dec 17,2025APIPolydimethylsiloxane, dihydroxy terminated enhances biocompatibility of polyurethanes, and improves epoxy resin hydrophobicity and toughness.....
Dec 18,2025APIFMOC-O-tert-Butyl-L-serine
71989-33-8You may like
FMOC-O-tert-Butyl-L-serine manufacturers
- Fmoc-O-tert-Butyl-L-serine
-

- $5.00 / 25kg
- 2025-12-18
- CAS:71989-33-8
- Min. Order: 1kg
- Purity: ≥99%
- Supply Ability: 100mt/year
- Fmoc-L-Ser(tBu)-OH
-
- $0.00/ kg
- 2025-12-18
- CAS:71989-33-8
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1T
- Fmoc-Ser(tBu)-OH
-

- $0.00 / 25Kg/Drum
- 2025-12-18
- CAS:71989-33-8
- Min. Order: 1KG
- Purity: 98%min
- Supply Ability: 500kgs