4-Chloro-7H-pyrrolo[2,3-d]pyrimidine: Applications in Medicinal Chemistry and its Improved Synthesis
May 11,2024
General Description
4-Chloro-7H-pyrrolo[2,3-d]pyrimidine is a key heterocyclic compound with a chlorine atom at the 4-position of its pyrimidine ring, offering unique properties for diverse applications. In medicinal chemistry, 4-Chloro-7H-pyrrolo[2,3-d]pyrimidine serves as a scaffold for developing potent kinase inhibitors, including promising candidates for cancer therapy and innovative treatments for inflammatory skin disorders like atopic dermatitis. Recent advancements in synthesis have led to improved methods with high yields, enhancing efficiency and accessibility for researchers. These developments underscore the compound's significance in drug discovery and organic synthesis, highlighting its potential in pharmaceutical research and materials science. Overall, 4-Chloro-7H-pyrrolo[2,3-d]pyrimidine plays a crucial role in advancing therapeutic interventions and organic compound synthesis, showcasing its versatility and importance in various scientific fields.
![Figure 1. 4-Chloro-7H-pyrrolo[2,3-d]pyrimidine.png Figure 1. 4-Chloro-7H-pyrrolo[2,3-d]pyrimidine.png](/NewsImg/2024-05-04/6385044591909133537333421.jpg)
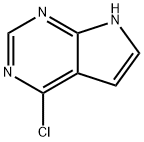
Figure 1. 4-Chloro-7H-pyrrolo[2,3-d]pyrimidine
Overview
4-Chloro-7H-pyrrolo[2,3-d]pyrimidine is a fascinating heterocyclic compound within the pyrimidine family, distinguished by its fused pyrrole and pyrimidine ring system, with a chlorine atom at the 4-position of the pyrimidine ring. This unique structure not only alters its chemical properties but also opens avenues for diverse applications. With its solubility in polar solvents and stability under specific conditions, it presents intriguing prospects in various fields. In pharmaceutical research, it holds potential as a precursor for synthesizing bioactive compounds due to its distinctive structure, which can influence pharmacokinetics and pharmacodynamics. Moreover, its utilization in materials science and organic electronics is gaining attention. In these domains, its electronic properties and compatibility with existing materials make it an attractive candidate for developing novel materials, such as organic semiconductors or ligands for catalysis. Synthesizing 4-Chloro-7H-pyrrolo[2,3-d]pyrimidine typically involves intricate multi-step organic reactions, necessitating precise control of reaction conditions to ensure high yields and purity. Despite the synthetic challenges, its versatility and potential applications make it a subject of continued interest and exploration in both academic and industrial research settings. 1
Applications in Medicinal Chemistry
4-Chloro-7H-pyrrolo[2,3-d]pyrimidine has emerged as a significant moiety in medicinal chemistry, particularly in the development of potent and selective inhibitors for various kinase targets. In the context of anticancer drug discovery, analogs of 4-Chloro-7H-pyrrolo[2,3-d]pyrimidine substituted with different amines have been identified as potent inhibitors of 3-Phosphoinositide-Dependent Kinase 1 (PDK1), a crucial target in cancer therapy. These analogs exhibit remarkable selectivity against PI3K/AKT-pathway kinases, making them promising candidates for anticancer agents. Moreover, the discovery of JTE-052 (Delgocitinib), a Janus Kinase (JAK) inhibitor bearing a highly three-dimensional spiro scaffold containing 4-Chloro-7H-pyrrolo[2,3-d]pyrimidine, represents a significant breakthrough in dermatological therapy. JTE-052 has demonstrated excellent physicochemical properties and efficacy in treating inflammatory skin disorders like atopic dermatitis. Its approval in Japan as the first drug among Janus kinase inhibitors underscores its potential as an effective and well-tolerated therapy for dermatological conditions, offering a promising alternative to conventional treatments like topical steroids, which often exhibit adverse side effects. In summary, the versatile applications of 4-Chloro-7H-pyrrolo[2,3-d]pyrimidine in medicinal chemistry range from anticancer agents targeting PDK1 to innovative therapies for inflammatory skin disorders, highlighting its importance as a scaffold in drug discovery and development. 2
Improved Synthesis
An enhanced method for synthesizing 4-chloro-7H-pyrrolo[2,3-d]pyrimidine has been developed, yielding a significant improvement with a 91% yield. The process involves starting with 6-chloro-5-(2,2-diethoxyethyl)-4-pyrimidinamine and utilizing hydrochloric acid in water as the solvent. The reaction proceeds by stirring the reactant mixture at 50°C for 4 hours in petroleum ether. After filtration, the solid crude product is obtained and further processed by triturating with a petroleum ether - EtOAc mixture to yield 4-chloro-7H-pyrrolo[2,3-d]pyrimidine. Moreover, an upgraded seven-step synthesis method has been devised, achieving a 31% overall yield starting from di-Me malonate. This approach significantly enhances the efficiency and practicality of synthesizing the 4-chloro-7H-pyrrolo[2,3-d]pyrimidine building block. The procedure is straightforward and operationally simple, making it a feasible option for researchers in need of this compound for various applications. This improved synthesis method represents a notable advancement in the field, providing a more efficient route to obtain 4-chloro-7H-pyrrolo[2,3-d]pyrimidine, a valuable intermediate in organic synthesis. 3
Reference
1. 4-Chloro-7H-pyrrolo[2,3-d]pyrimidine. National Center for Biotechnology Information. 2024; PubChem Compound Summary for CID 5356682.
2. Murphy ST, Alton G, Bailey S, et al. Discovery of novel, potent, and selective inhibitors of 3-phosphoinositide-dependent kinase (PDK1). J Med Chem. 2011; 54(24): 8490-8500.
3. Zhang YL. An improved synthesis of 4-chloro-7H-pyrrolo[2,3-d]pyrimidine. Chemistry of Heterocyclic Compounds. 2018; 54(1): 638–642.
- Related articles
- Related Qustion
- 4-Chloro-7H-pyrrolo[2,3-d]pyrimidine: properties, applications and safety Oct 10, 2023
4-Chloro-7H-pyrrolo[2,3-d]pyrimidine is a versatile compound used in pharmaceutical synthesis with potential applications in disease treatment.
- 4-Chloro-7H-pyrrolo[2,3-d] pyrimidine— a medical intermediate Dec 18, 2019
Being an important intermediate in medicine, 4-Chloro-7H-pyrrolo[2,3-d] pyrimidine had been widely used in the synthesis of many pharmaceutical intermediates at home and abroad.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringDicyclohexylamine inhibits bacterial growth via spermidine synthase, showing sensitivity in Streptococcus uberis, with potential therapeutic applications and LC-MS/MS detection in honey.....
May 11,2024API4-Chloro-7H-pyrrolo[2,3-d]pyrimidine
3680-69-1You may like
4-Chloro-7H-pyrrolo[2,3-d]pyrimidine manufacturers
- 4-Chloro-7H-pyrrolo[2,3-d]pyrimidine
-
![3680-69-1 4-Chloro-7H-pyrrolo[2,3-d]pyrimidine](/ProductImageEN1/2025-04/Small/173d652e-8715-4c8e-9ec4-952b07211e4b.gif)
- 2025-12-18
- CAS:3680-69-1
- Min. Order:
- Purity: 0.99
- Supply Ability:
- 4-Chloropyrrolo[2,3-d]pyrimidine
-
![3680-69-1 4-Chloropyrrolo[2,3-d]pyrimidine](https://img.chemicalbook.com/ProductImageEN/2019-3/Small/f0c7e8e7-13cd-4ffd-9857-c465e8e31b7f.jpg)
- $22.00 / 1KG
- 2025-12-18
- CAS:3680-69-1
- Min. Order: 100 mkg
- Purity: 99%
- Supply Ability: 20mt
- 4-Chloro-7H-pyrrolo[2,3-d]pyrimidine
-
![3680-69-1 4-Chloro-7H-pyrrolo[2,3-d]pyrimidine](/ProductImageEN/2022-05/Small/a95027d5-b911-4ec6-9069-319704d850ff.jpg)
- $0.00 / 1kg
- 2025-12-18
- CAS:3680-69-1
- Min. Order: 1kg
- Purity: 98%min HPLC
- Supply Ability: 1000kg