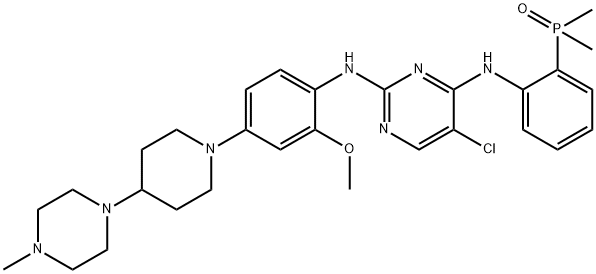
Brigatinib: mechanism of action and pharmacokinetics and side effects
Jul 12,2023
![]() General Description
General Description
Brigatinib, sold under the brand name Alunbrig among others, is a small-molecule targeted cancer therapy being developed by Ariad Pharmaceuticals, Inc. Brigatinib acts as both an anaplastic lymphoma kinase (ALK) and epidermal growth factor receptor (EGFR) inhibitor1. It is a treatment for a type of lung cancer called non-small cell lung cancer (NSCLC) that has spread to other parts of the body (advanced or metastatic NSCLC). Adverse events associated with Brigatinib include nausea, diarrhea, fatigue, cough, interstitial lung disease, hypertension, visual disturbance, and laboratory abnormalities. It is important to note the warnings and precautions for potential pulmonary adverse reactions, hypertension, cardiac issues, and embryo-fetal toxicity.
Figure 1. Tablet of brigatinib
![]()
![]() Mechanism of action
Mechanism of action
Brigatinib is a potent tyrosine kinase inhibitor that demonstrates its therapeutic efficacy through the inhibition of various kinases involved in cancer progression. It targets key proteins such as anaplastic lymphoma kinase (ALK), ROS1, insulin-like growth factor 1 receptor (IGF-1R), fms-like tyrosine kinase 3 (FLT-3), and certain epidermal growth factor receptor (EGFR) mutations. By effectively blocking the phosphorylation of ALK, Brigatinib disrupts the activation of downstream signaling pathways crucial for tumor growth and survival. Furthermore, its inhibitory actions on ROS1, IGF-1R, FLT-3, and EGFR aberrations further contribute to its broad anticancer activity. Through this multifaceted kinase inhibition, Brigatinib exhibits comprehensive therapeutic potential against various malignancies. Its ability to impede ALK signaling pathways not only curtails tumor proliferation but also hinders angiogenesis and metastasis process. Additionally, the suppression of ROS1, IGF-1R, FLT-3, and EGFR aberrant signaling cascades contributes to the overall efficacy of Brigatinib in combating cancer by reducing cellular proliferation, promoting apoptosis, and thwarting resistance mechanisms. Overall, the distinct mechanism of action demonstrated by Brigatinib, involving the inhibition of multiple kinases, provides a robust foundation for its efficacy in targeted cancer therapy. By selectively targeting these pivotal proteins and disrupting their signaling, Brigatinib holds promise in the treatment of diverse malignancies, improving patient outcomes and potentially overcoming resistance to conventional therapies. 1
Pharmacokinetic
After single oral doses of brigatinib ranging from 30 to 240 mg, the median time to reach maximum concentration (Cmax) ranged between 1 and 4 hours. Administration of brigatinib with a high-fat meal showed a 13% reduction in Cmax compared to fasting conditions, with no effect on AUC. Brigatinib exhibits approximately 66% binding to human plasma proteins, and this binding is not concentration-dependent. The blood-to-plasma concentration ratio is 0.69. The mean apparent steady-state volume of distribution is 153 L after oral administration of 180 mg brigatinib once daily. The mean apparent oral clearance of brigatinib at steady state is 12.7 L/h, and the mean plasma elimination half-life is 25 hours. The primary metabolic pathways for brigatinib involve CYP2C8 and CYP3A4 enzymes. N-demethylation and cysteine conjugation are the major metabolic pathways identified. After oral administration of a radiolabeled 180 mg brigatinib dose, unchanged brigatinib and its primary metabolite AP26123 accounted for 92% and 3.5% of circulating radioactivity, respectively. Approximately 65% of the administered dose was recovered in feces, while 25% was recovered in urine. Exposure to brigatinib appears similar between individuals with mild hepatic impairment and those with normal hepatic function. Factors such as age, race, sex, body weight, albumin concentration, and mild-to-moderate renal impairment do not have clinically meaningful effects on the pharmacokinetics of brigatinib. However, the pharmacokinetic properties of brigatinib have not been studied extensively in patients with moderate-to-severe hepatic impairment or severe renal impairment. 2
Side effect
Adverse events reported in patients treated with brigatinib include nausea, diarrhea, vomiting, constipation, abdominal pain, fatigue, pyrexia, cough, dyspnea, interstitial lung disease (ILD)/pneumonitis, hypoxia, headache, peripheral neuropathy, rash, hypertension, muscle spasms, back pain, myalgia, arthralgia, pain in extremity, decreased appetite, visual disturbance, pneumonia, and insomnia. Laboratory abnormalities include increased AST, hyperglycemia, increased CPK, increased lipase, increased ALT, increased amylase, increased alkaline phosphatase, decreased phosphorus, prolonged activated partial thromboplastin time, anemia, and lymphopenia. Brigatinib carries warnings and precautions for potentially severe and fatal pulmonary adverse reactions consistent with ILD/pneumonitis, hypertension, bradycardia, visual disturbance, CPK elevation, pancreatic enzyme elevation, hyperglycemia, and embryo-fetal toxicity. 3
Reference
1. Huang WS, Liu S, Zou D, et al. Discovery of Brigatinib (AP26113), a Phosphine Oxide-Containing, Potent, Orally Active Inhibitor of Anaplastic Lymphoma Kinase. J Med Chem. 2016, 59(10):4948-4964.
2. ARIAD Pharmaceuticals prescribing information. Inc. ALUNBRIG? (brigatinib): US 2017.
3. Markham A. Brigatinib: First Global Approval. Drugs, 2017, 77(10):1131-1135.
- Related articles
- Related Qustion
- Brigatinib: Indications, Dosing, Mechanism of Action and Side Effects Dec 16, 2024
Brigatinib is a new generation anaplastic lymphoma kinase (ALK) inhibitor.
- Brigatinib: Development History and Preclinical Studies Nov 19, 2024
Brigatinib, a crucial treatment for NSCLC patients with EGFR and ALK mutations, effectively inhibits ALK with unique features, overcoming resistance and offering hope in lung cancer treatment.
- The warnings and precautions of Brigatinib Mar 6, 2024
ALUNBRIG (Brigatinib) is a kinase inhibitor indicated for the treatment of adult patients with anaplastic lymphoma kinase (ALK)-positive metastatic non-small cell lung cancer (NSCLC) as detected by an FDA-approved test.
N-Phenyl-bis(trifluoromethanesulfonimide) is a stable, non-reactive solid used in organic synthesis and battery production with handling precautions.....
Jul 12,2023APIRecent scientific research has shed light on the toxic effects and reproductive disruptions caused by a commonly used compound, bis(4-hydroxyphenyl) sulfone.....
Jul 12,2023APIBrigatinib
1197953-54-0You may like
- Brigatinib
-

- $0.00 / 1kg
- 2025-12-12
- CAS:1197953-54-0
- Min. Order: 1kg
- Purity: 99.0%
- Supply Ability: 10000
- Brigatinib
-
- $0.00 / 25kg
- 2025-12-01
- CAS:1197953-54-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10000KGS
- Brigatinib USP/EP/BP
-
- $1.10 / 1g
- 2025-11-18
- CAS:1197953-54-0
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons min