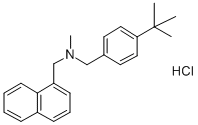
Butenafine Hydrochloride: Antifungal Mechanism, SLN Formulation, Tinea Pedis Efficacy, and Skin Sample Assessment
Aug 27,2025
Butenafine hydrochloride is a topical antifungal medicine used to treat fungal skin infections such as athlete's foot, jock itch, and ringworm. Butenafine Hydrochloride is the hydrochloride salt form of butenafine, a synthetic benzylamine derivative with fungicidal properties. Butenafine hydrochloride interferes with the biosynthesis of ergosterol, an important component of fungal cell membranes, by inhibiting the epoxidation of squalene. This alters fungal membrane permeability and causes growth inhibition. Butenafine hydrochloride is active against a number of dermatophytes, including Trichophyton rubrum, Trichophyton mentagrophytes, Microsporum canis, Sporothrix schenckii, and yeasts, including Candida albicans and C. parapsilosis. It has a role as an EC 1.14.13.132 (squalene monooxygenase) inhibitor and an antifungal drug. It is a hydrochloride and an ammonium salt. It contains a butenafine.

Formulation and Evaluation of Butenafine Hydrochloride-Incorporated Solid Lipid Nanoparticles
Fungal infections are one of the most common skin diseases in the global population. According to an unpublished survey by the International Foundation of Dermatology, superficial mycosis was usually reported as one of the three most common diseases among the community patterns of skin diseases in nine different countries across the world. Butenafine hydrochloride (BUTE) is a synthetic allylamine antifungal agent. The suggested mode of action is that inhibits the enzyme squalene monooxygenase, which is responsible for converting squalene to 2,3-oxide squalene. Hence, it interferes with the biosynthesis of ergosterol, resulting in increased cellular permeability and leakage of cellular contents. Blockage of squalene monooxygenase causes squalene accumulation and leads to a fungicidal effect. The objective of this study was to develop and evaluate antifungal drug Butenafine hydrochloride-SLNs for treating topical fungal infections. The novelty of this work is the use of novel excipients such as natural surfactants and lipids, the use of organic solvents in the fabrication of SLN, and the very simple and novel modified solvent emulsification method of preparation. The involvement of organic solvents in the Butenafine hydrochloride -SLN formulation was a possible explanation for the increase in surfactant concentration, which contributed to a small increase in particle size.[1]
Various formulation ingredients like lipid, surfactant and DMSO have an influence on the fabrication of SLN dispersions, which directly affects the particle size, EE, and ZP of SLN. The DSC and XRD studies indicated that the optimized formulation was amorphous in nature and contained the drug, DMSO, and surfactant entrapped inside the SLN. Rheological and spreadability study of Butenafine hydrochloride -SLN aloe vera gel revealed excellent slip properties beneficial for inflamed skin application. Along with its excellent rheological and spreadability, the BUTE-SLN gel possesses good occlusive properties and hydrating potential. For long-term drug delivery through the stratum corneum, BUTE-SLN showed accumulation of the SLN in the stratum corneum with controlled drug release. Butenafine hydrochloride -SLN demonstrated increased skin deposition, improved in vitro antifungal action, and a topical formulation that was less irritating. The skin irritation study revealed the non-irritant nature of BUTE-SLN gel, which explains its usefulness in treating fungal infections. As a result, the Butenafine hydrochloride -SLN aloe vera gel formulation is a cost-effective, novel, and reproducible alternative to the currently available traditional dosage method.
Butenafine hydrochloride: for the treatment of interdigital tinea pedis
Butenafine hydrochloride, a derivative of benzylamine with potent fungicidal activity is a new generation of antimycotic compound that has shown to be extremely effective against experimentally-induced tinea pedis in the guinea-pig, a situation that resembles synergetic pathology similar to that of tinea pedis in humans. Butenafine hydrochloride, (N-4-tert-butylbenzyl-N-methyl-1-naphthalenemethyl-amine hydrochloride) with a chemical structure and mode of action similar to those of the allylamines, demonstrates superior fungicidal activity in vitro against dermatophytes and superior fungistatic activity toward Candida albicans than that of naftifine and terbinafine.[2]
In vitro, pharmacodynamic data has shown that the geometric mean of minimum inhibitory concentration values for butenafine were comparatively lower than those of naftifine and clotrimazole against clinical isolates for many dermatophytes. It inhibits sterol synthesis by blocking the squalene epoxidation stage in fungi. In phramacokinetic assessments butenafine achieves and maintains high concentrations and long retention time in skin, with associated anti-inflammatory activity in vivo. In controlled clinical trials when applied topically, butenafine appears to be well tolerated with a subjective mild burning sensation at the application site. There were no withdraws from the study. Butenafine hydrochloride is sparingly soluble in water but readily soluble in methanol, ethanol, dichloromethane and chloroform. If incorporated properly in semisolid topical preparations, with a balanced vehicle, butenafine hydrochloride potentially exhibits as a promising alternative antimycotic agent for the treatment of tinea pedis.
A simple and rapid method to assess butenafine hydrochloride in skin samples
Butenafine (N-4-tert-butylbenzyl-N-methyl-1-naphthalenemethylamine hydrochloride), a broad-spectrum topical antifungal agent, shows excellent therapeutic efficacy in humans with dermatomycoses. The drug readily interacts with lipids and is incorporated into membrane phospholipids, which may explain the excellent antifungal efficacy and long duration of action when it is used as a topical antifungal agent in humans. The drug mechanism of action is based on the inhibition of the fungal enzyme squalene epoxidase, blocking the biosynthesis of ergosterol, which is an essential component of fungal cell membranes. Butenafine hydrochloride (BTF) was introduced to the Brazilian market in 2007 formulated as a 1% (w/w) cream.[3]
Butenafine hydrochloride solubility in the receptor phase was determined, achieving sink conditions (the total quantity of BTF applied on the donor compartment was soluble in less than one-third of the receptor media), to have a high capacity to dissolve and carry away the drug. After 8 h of experiment, the skin was removed from the Franz diffusion cell and the excess of formulation was removed with the help of a spatula followed by the separation of the dermis from the epidermis using a scalpel. The skin layers were weighted and BTF was extracted according to the butenafine hydrochloride extraction procedure previously described. The developed performance test for butenafine hydrochloride from creams enclosing the use of Franz diffusion cell with quantification of the drug in the main layers of the skin and in the receptor compartment has the necessary characteristics for a good bioanalytical method: reproducibility, reliability, ability to measure drug release from the finished dosage form and capacity for measuring changes in drug release characteristics from the finished product.
References
[1]Baviskar A, Kashid V, Ahirrao S, Bhambere D, Akul M. Formulation and Evaluation of Butenafine Hydrochloride-Incorporated Solid Lipid Nanoparticles as Novel Excipients for the Treatment of Superficial Fungal Infections. Turk J Pharm Sci. 2024 Sep 2;21(4):313-326. doi: 10.4274/tjps.galenos.2023.80799. PMID: 39224083; PMCID: PMC11589089.
[2]Syed, T A, and H I Maibach. “Butenafine hydrochloride: for the treatment of interdigital tinea pedis.” Expert opinion on pharmacotherapy vol. 1,3 (2000): 467-73. doi:10.1517/14656566.1.3.467
[3]Barth, Aline Bergesch et al. “A simple and rapid method to assess butenafine hydrochloride in skin samples and a comparative cutaneous retention study of two marketed formulations.” Biomedical chromatography : BMC vol. 25,10 (2011): 1132-7. doi:10.1002/bmc.1582
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical Engineering2,2-Bis(bromomethyl)propane-1,3-diol, used in resins and foams, shows carcinogenic effects in animals and potential reproductive toxicity.....
Aug 28,2025Chemical ReagentsButenafine hydrochloride
101827-46-7You may like
- Clinical Application Research of Sertraline
Dec 19, 2025
- Nandrolone: brief history and application research
Dec 18, 2025
- Application research of Ceftiofur hydrochloride
Dec 18, 2025
Butenafine hydrochloride manufacturers
- Butenafine hydrochloride
-
- $0.00 / 1kg
- 2025-12-18
- CAS:101827-46-7
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: Customise
- Butenafine hydrochloride
-

- $0.00 / 1Kg/Bag
- 2025-12-18
- CAS:101827-46-7
- Min. Order: 1KG
- Purity: 99%-101%;JP
- Supply Ability: 500KGS
- Butenafine hydrochloride
-

- $53.00 / 500mg
- 2025-12-10
- CAS:101827-46-7
- Min. Order:
- Purity: 100.00%
- Supply Ability: 10g