Chemical and physical properties of Sulfur hexafluoride
Dec 21,2023
Overview
Sulfur hexafluoride (SF6) is the greenhouse gas (GHG) with the largest known 100-year global warming potential (GWP) and an atmospheric lifetime of 580–3200 years. SF6 is primarily used in electrical circuit breakers and high-voltage gas-insulated switchgear in electric power transmission and distribution (ETD) equipment; its emissions occur during manufacturing, use, servicing, and disposal. There is also usage and associated emissions of SF6 from producing magnesium and electronics. Because of its huge GWP and long atmospheric lifetime, emissions of SF6 accumulate in the atmosphere and will influence Earth's climate for thousands of years[1].
Properties
Sulfur hexafluoride is an organic, colorless, odorless, noninflammable, nontoxic, and long-lived (atmospheric lifetime of 800–3200 years) gas. Its structure consists of six fluorine atoms bonded with one sulfur atom at the center. Sulfur hexafluoride melts at − 50.5 °C and sublimes at − 63.9 °C, critical pressure 3.759 mpa (37.2 atm), critical temperature, 45.55 °C, liquid density 2.863 g/cm3, gas density 1.336 g/cm3, dielectric constant 1.00204 (gas), 1.81 (liquid)[2-3].
It is an excellent dielectric because a high gas density can be maintained at low temperatures, and all the electrons are tightly bound. It is an excellent electron scavenger and has strong breakdown strength. During electrical breakdown, it captures primary electrons, forming SF5− or SF6− ions and F atoms. In addition, In sulfur hexafluoride, the sulfur atom is shielded by six fluorine atoms, which impede kinetically any reaction with water, alkali hydroxides, ammonia, or strong acids; as a result, it remains inert to these reagents.
Polar or Non-polar
In the sulfur hexafluoride (SF6) molecule, sulfur (S) occupies the central position as it is less electronegative than the surrounding fluorine (F). Sulfur has six electrons in its valence shell. Fluorine needs only one electron to complete its octet. Therefore, sulfur will share all six electrons with the fluorine atoms, resulting in six single covalent bonds. The fluorine atoms are placed symmetrically around sulfur, creating an octahedral structure.
Fluorine (E.N =3.98) is more electronegative than sulfur (E.N = 2.58). An electronegativity difference of 1.4 units is present between the two bonded atoms. So, F strongly attracts the shared electron cloud from each S-F bond in the SF6 molecule. Oppositely charged poles develop in the molecule as each F atom gains a partial negative (Fδ-) charge while the central S atom attains a dense partial positive (Sδ+) charge. Consequently, each S-F bond in the SF6 molecule is polar. This is called the bond polarity of SF6.
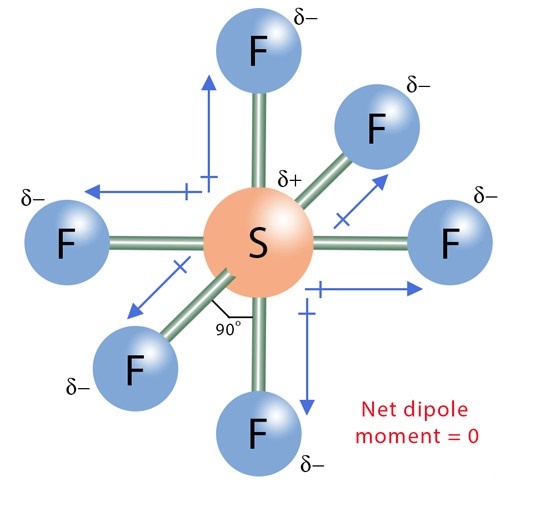
As each S-F bond in the SF6 molecule is polar al,l S-F bonds also have a specific dipole moment value. The dipole moment of each S-F bond points from the Sδ+ to Fδ-.
According to the Valence Shell Electron Pair Repulsion (VSEPR) theory of chemical bonding, SF6 is an AB6-type molecule. To one atom of sulfur in the center, six bond pairs are attached, and there is no lone pair on the central atom. The geometry of the molecule is octahedral. The octahedral molecular geometry of SF6 is perfectly symmetrical. The individual dipole moments of polar S-F bonds get canceled in opposite directions. Thus, SF6 is a non-polar molecule with a zero net dipole moment.
References
[1] Mishra, Arti et al. “Influence of greenhouse gases on plant epigenomes for food security.” Biomass, Biofuels, Biochemicals 14 1 (2022).
[2] Meshri, D. “Chapter 20 – Industrial Applications of Inorganic Fluorides.”Advanced Inorganic Fluorides 2000.
[3] Lei Hu. “Declining, seasonal-varying emissions of sulfur hexafluoride from the United States.” Atmospheric Chemistry and Physics (2023).
- Related articles
- Related Qustion
- How are SF6 Lewis structures and hybrids formed Nov 24, 2023
The SF6 Lewis structure consists of a central atom, sulphur (S), and six outer atoms, fluorine (F). The sulphur atom (S) and each fluorine atom (F) are connected by a single bond, with three lone pairs of electrons on each fluorine atom, sy
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringDoravirine is a novel nonnucleoside reverse transcriptase inhibitor (NNRTI) currently in development for the treatment of patients with human immunodeficiency virus type 1 (HIV-1) infection in combination with other antiretroviral therapies....
Dec 21,2023Biochemical EngineeringSulfur hexafluoride
2551-62-4You may like
Sulfur hexafluoride manufacturers
- Sulfur hexafluoride
-

- $1.00 / 1kg
- 2019-07-06
- CAS:2551-62-4
- Min. Order: 1 g
- Purity: 99%
- Supply Ability: 100KG