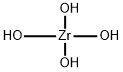
Zirconium Hydroxide: Advanced Material Applications
Dec 10,2025
Zirconium hydroxide is a white to off-white solid that is sparinglysoluble in water. It has relatively high specific surface area, ion exchange capacity, good thermalstability, and dielectric properties. Zirconium hydroxide can be used as: A starting material to prepare sulfated zirconia catalyst for liquid-phase dehydration of sorbitol; to fabricate coated magnetic materials for the removal of anionic dyes from solution. Zirconium Hydroxide is a highly water insoluble crystalline Zirconium source for uses compatible with higher (basic) pH environments. Hydroxide, the OH- anion composed of an oxygen atom bonded to a hydrogen atom, is commonly present in nature and is one of the most widely studied molecules in physical chemistry. Hydroxide compounds have diverse properties and uses, from base catalysis to detection of carbon dioxide. In a watershed 2013 experiment, scientists at JILA achieved evaporative cooling of compounds for the first time using hydroxide molecules, a discovery that may lead to new methods of controlling chemical reactions and could impact a range of disciplines, including atmospheric science and energy production technologies.

High-efficiency air filter paper loaded with reactive zirconium hydroxide
Since the chlorine attack in the Battle of Ypres during World War I, chemical weapons began to be used on a large scale on the battlefield, and protective equipment against chemical weapons came into existence. Zirconium hydroxide (ZH) is an amorphous porous reagent with both acid–base and redox properties, which is benefited from multifarious active centers, for instance, coordinated unsaturated metal cations (Zrδ+), oxygen vacancies, bridging hydroxyl groups (b-OH) showing Brønsted acidity, and terminal hydroxyl groups (t-OH) showing Brønsted basicity. Excellent adsorption performance is obtained due to its porous structure, which can be combined with activated carbon as an adsorbent to adsorb persistent anionic reactive dyes and water pollutants. Variable-temperature in situ attenuated total reflection (ATR) infrared spectroscopy was employed to study the reaction kinetics of dimethyl methylphosphonate (DMMP) by Zirconium hydroxide in the ρ(PCH3) mode. It was found that both adsorption and decomposition processes of DMMP occurred and the pseudo-second-order model best fitted the reaction data. The purpose of this paper is to explore and develop a methodology for producing self-decontaminating air filter paper (SD-AFP), which can maintain high filtration efficiency, low pressure drop and moderate tensile strength of AFP as well as degrade HD and VX simultaneously.[1]
In this work, a self-decontaminating air filter paper that can simultaneously degrade HD and VX has been successfully prepared using zirconium hydroxide as a decontaminant and an intra-pulp addition technology. Based on FE-SEM, EDX, XRD and TGA analysis, it was found that ZH was uniformly distributed and attached in the interstices of the glass fiber. Compared to the base paper and ZH powder, the glass fiber and ZH used for the composite material had no obvious crystalline changes, and Zirconium hydroxide had no distinct aggregation. Taking filtration efficiency, pressure drop and tensile strength as evaluation indicators, the influence of the fiber grammage, amount of adhesive and retention of Zirconium hydroxide on the general performance of SD-AFP was investigated. The SD-AFP with a fiber grammage of 50 g m−2 and the addition of adhesive of 2% had high filtration efficiency, low breathing pressure drop and moderate tensile strength, which was used to conduct degrading experiment. It was discovered that HD and VX were both converted into non-toxic or low-toxic products through the degradation effect research. The half-lives of HD and VX on SD-AFP were 2.6 h and 16.2 min, respectively. It is displayed that the in-pulp addition technology of preparing SD-AFP is feasible, which have achieved the aim to degrade HD and VX. Further research will be devoted to the exploitation of new technology and the optimization of technological conditions to decrease the pressure drop.
Battling Chemical Weapons with Zirconium Hydroxide Nanoparticle Sorbent
There are many technical challenges regarding real-world toxic chemical sequestration and decontamination, including the diversity of toxic chemicals, multiple decomposition routes, and complexity of environmental contaminants that may poison or alter reaction pathways and kinetics of catalysts and sorbents. Of the explored sorbent systems, zirconium hydroxide (Zr(OH)4, ZH) has emerged as a front-runner in chemical decontamination by demonstrating rapid sequestration and decomposition of a variety of toxic industrial chemicals (TICS, particularly acid gases such as SO2 and NO2) and CWAs (VX, soman, and sulfur mustard [bis(2-chloroethyl) sulfide, HD]). Rapid sequestration and decomposition activity are linked to the amorphous and structurally defective nature of Zirconium hydroxide, giving rise to coordinatively unsaturated (cus) Lewis acidic Zr4+/3+ sites and high concentrations of reactive hydroxyl species. This work identifies the surface reactivity, stability, and speciation on ZH under ambient conditions when exposed to CO2, SO2, and NO2. In situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) identified surface adsorbates on ZH nanopowders under pseudo-operando conditions at ambient pressure. Predosing Zirconium hydroxide with environmental contaminants followed by GB exposure enabled direct binding strength comparisons of the adsorbates. Complementary density functional theory (DFT) calculations with cluster models were employed to bolster the experimentally determined reaction mechanisms and provide adsorption entropies, enthalpies, and bond lengths.[2]
Zirconium hydroxide (ZH) nanopowder, a promising sorbent and decontaminant, was predosed with CO2, SO2, and NO2 before exposure to the chemical warfare agent sarin (GB). All dosed gases rapidly formed adsorbates on ZH including (bi)carbonates, sulfites, sulfates, nitrites, nitrates, and other unconfirmed species. GB dosed on clean ZH showed immediate decomposition of GB to the hydrolysis product IMPA. The reaction continues with time on the ZH surface to MPA and possibly PA. On contaminated surfaces, decomposition product formation was impeded as evidenced by the accumulation of intact GB using in situ DRIFTS. However, no indication of GB was observed upon ex situ examination with XPS. XPS data of all GB-dosed Zirconium hydroxide samples showed hydrolysis of the fluorine bond to form an inorganic-like fluoride species, indicative of GB decomposition. Microbreakthrough capacity measurements indicate that ZH has a 7-fold increased capacity for NO2 vs SO2. DFT predicted that NO2 is unlikely to react with the Zirconium hydroxide surface and must either dimerize to N2O4 or react with adsorbed surface water to form the more reactive HNO3 or HONO species. Additionally, the nitrite and nitrate adsorbates were calculated to react with only a single hydroxyl site compared to two hydroxyl sites for SO2 adsorbates.
Environmental Effects on Zirconium Hydroxide
Zirconium hydroxide (Zr(OH)4) has excellent sorption properties and wide-ranging reactivity toward numerous types of chemical warfare agents (CWAs) and toxic industrial chemicals. Under pristine laboratory conditions, the effectiveness of Zr(OH)4 has been attributed to a combination of diverse surface hydroxyl species and defects; however, atmospheric components and trace contaminants can form adsorbates with potentially detrimental impact to the chemical reactivity of Zirconium hydroxide. Here, we report the hydrolysis of a CWA simulant, dimethyl methylphosphonate (DMMP) on Zr(OH)4 determined by gas chromatography–mass spectrometry and in situ attenuated total reflectance Fourier transform infrared spectroscopy under ambient conditions. DMMP dosing on Zr(OH)4 formed methyl methylphosphonate and methoxy degradation products on free bridging and terminal hydroxyl sites of Zr(OH)4 under all evaluated environmental conditions. CO2 dosing on Zr(OH)4 formed adsorbed (bi)carbonates and interfacial carbonate complexes with relative stability dependent on CO2 and H2O partial pressures. High concentrations of CO2 reduced DMMP decomposition kinetics by occupying Zr(OH)4 active sites with carbonaceous adsorbates. Elevated humidity promoted hydrolysis of adsorbed DMMP on Zirconium hydroxide to produce methanol and regenerated free hydroxyl species. Hydrolysis of DMMP by Zr(OH)4 occurred under all conditions evaluated, demonstrating promise for chemical decontamination under diverse, real-world conditions.[3]
References
[1]Huang X, Zhao T, Zhang H, Yan C, Sha J, Tang H, Zhu H, Wu Y. Dual-purpose high-efficiency air filter paper loaded with reactive zirconium hydroxide for the filtration aerosols and degradation of chemical warfare agents. RSC Adv. 2021 Nov 1;11(56):35245-35257. doi: 10.1039/d1ra06903a. PMID: 35493143; PMCID: PMC9042809.
[2]Balow, Robert B et al. “Battling Chemical Weapons with Zirconium Hydroxide Nanoparticle Sorbent: Impact of Environmental Contaminants on Sarin Sequestration and Decomposition.” Langmuir : the ACS journal of surfaces and colloids vol. 37,23 (2021): 6923-6934. doi:10.1021/acs.langmuir.1c00380
[3]Balow, Robert B et al. “Environmental Effects on Zirconium Hydroxide Nanoparticles and Chemical Warfare Agent Decomposition: Implications of Atmospheric Water and Carbon Dioxide.” ACS applied materials & interfaces vol. 9,45 (2017): 39747-39757. doi:10.1021/acsami.7b10902
- Related articles
- Related Qustion
Cholesterol is either synthesised de novo in our cells or is taken in the form of dietary cholesterol from food sources. This article will introduce its synthesis pathway in human body.....
Dec 9,2025Biochemical EngineeringAlfacalcidol treats secondary hyperparathyroidism/osteoporosis by regulating calcium-phosphorus metabolism, with combinations enhancing efficacy.....
Dec 10,2025APIZirconium hydroxide
14475-63-9You may like
Zirconium hydroxide manufacturers
- Zirconium hydroxide
-

- $0.00 / 50g
- 2025-08-22
- CAS:14475-63-9
- Min. Order: 50g
- Purity: 99%
- Supply Ability: 500kg
- Zirconium hydroxide
-

- $30.00 / 1KG
- 2025-06-27
- CAS:14475-63-9
- Min. Order: 50KG
- Purity: 99%
- Supply Ability: 500000kg
- Zirconium hydroxide
-

- $0.00 / 1kg
- 2025-06-20
- CAS:14475-63-9
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 20 tons