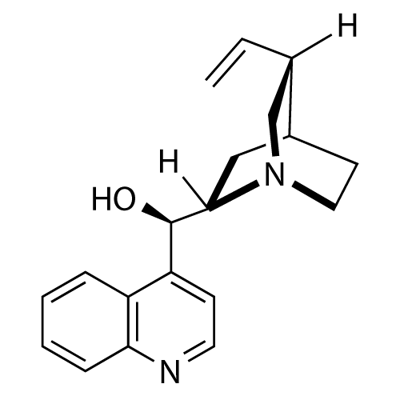
Cinchonidine: Synthesis, Applications in Anti-malarial Research and Catalysis
Apr 23,2025
Cinchonidine is 8-epi-Cinchonan in which a hydrogen at position 9 is substituted by hydroxy (R configuration). A diasteroisomer of cinchonine, it occurs in the bark of most varieties of Cinchona shrubs, and is frequently used for directing chirality in asymmetric synthesis.

Click Inspired Synthesis of Novel Cinchonidine Glycoconjugates
Among all the malaria parasites, P. falciparum is the most predominant species which has developed drug resistance against most of the commercial anti-malarial drugs. Thus, finding a new molecule for the inhibition of enzymes of P. falciparum is the pharmacological challenge in present era. Herein, ten novel molecules have been designed with an amalgamation of cinchonidine, carbohydrate moiety and triazole ring by utilizing copper-catalyzed click reaction of cinchonidine-derived azide and clickable glycosyl alkynes. The molecular docking of developed molecules showed promising results for plasmepsin inhibition in the form of effective binding with target proteins. Cinchona alkaloids are cheap natural source of anti-malarial activity which provides opportunity towards development of new anti-malarial drug leads by synthetic modifications in their chemical structures. The anti-malarial activities of the four major alkaloids of this category follows the order Quinidine > Quinine > Cinchonidine > Cinchonine9. Thus, according to their anti-malarial activities, quinidine and quinine are the most interesting alkaloids to start with. But high anti-arrhythmic activities of these two alkaloids limit their use as regular anti-malarial drug because of cardiac risks even with smaller doses. Also, the drug resistance to quinine curbs the prospects of using this moiety in new drug leads. Therefore, cinchonidine remains the next potential molecule which can be modified structurally to enhance its anti-malarial activity.[1]
With a motive to develop novel cinchona alkaloid conjugates with anti-malarial activity, we preferred cinchonidine as the suitable alkaloid. Carbohydrate scaffold was chosen as a potential protein-binding moiety and 1,2,3-triazole was taken as suitable pharmacophore spacer to enhance activity of the resulting molecule. The designed molecules containing these three moieties were selected as cinchonidine glycoconjugates with a triazole linker. Cu-Catalyzed click reaction is a facile and high yielding approach for 1,3-dipolar cycloaddition of organic azides and terminal alkynes which goes well with carbohydrate moiety and has produced very interesting glycosyl triazoles with various applications. Thus, in continuation of our previous experience on click chemistry in glycoscience, this reaction was chosen for cycloaddition reaction of cinchonidine-derived azide and sugar-derived terminal alkynes to achieve our designed target molecules. The strategy for synthesis of cinchonidine-glycoconjugates with a triazole linker was initiated with the synthesis of azido-derivative of cinchonidine. The free hydroxy group at C-9 of cinchonidine was chosen to be converted into azide group. Simple mesylation of the hydroxy group by treatment with methanesulphonyl chloride in presence of triethylamine afforded O-mesylated cinchonidine derivative which was as such subjected to heating with sodium azide in aqueous DMF and afforded 9-epi-9-azido-9-deoxycinchonidine. The azido derivative was characterized by NMR, MS, and IR spectroscopy.
Ten novel molecular sets consisting of cinchonidine, triazole ring and carbohydrate scaffolds were designed and synthesized using Cu-catalyzed azide-alkyne cycloaddition reaction of 9-epi-9-azido-9-deoxycinchonidine with ten different glycosyl O-propargyl ethers. Developed cinchonidine glycoconjugates were subjected to docking studies for the evaluation of the interaction probability for anti-malarial activity with appreciable values of inhibitory effect. The molecular set has been subjected to both fragment docking and whole molecular docking using AutoDock and AutoDock Vina softwares. The detailed comparative docking study revealed that moieties are showing H-bond interaction at the target site where the anti-malarial active co-crystal moiety, R367 has been reported to show its interaction with amino acid residues of plasmepsin enzyme in the crystal structure.
Plausible Pnicogen Bonding of epi-Cinchonidine as a Chiral Scaffold in Catalysis
As a non-covalent interaction of a chiral scaffold in catalysis, pnicogen bonding of epi-cinchonidine (epi-CD), a cinchona alkaloid, was simulated to consider whether the interaction can have the potential controlling enantiotopic face like hydrogen bonding. In this paper, we studied the possibility of pnicogen bonding between a Lewis base of epi-cinchonidine (epi-CD), a type of cinchona alkaloid, and covalently bonded P, As, Sb, and Bi of the pnictide family as a Lewis acid. To our knowledge, there are no reports on pnicogen bonding in any chiral scaffold (among chiral ligands, chiral auxiliaries, and chiral catalysts) like cinchona alkaloids so that a theoretical study on pnicogen bonding can be a guidance for reaction designs in the field of catalysis and asymmetric synthesis.[2]
Firstly, the binding characteristics of the analytes, phosphane derivatives complexed with epi-Cinchonidine, were investigated. They interacted with two functional groups of epi-CD, the hydroxyl group (X-epi-CD1) and the quinoline ring (X-epi-CD2). The distances of the pnicogen bonding are ca. 2.6–3.6 Å between the hydroxyl group and phosphorus in X-epi-CD1 and ca. 2.1−4.0 Å between quinoline nitrogen (N17) and phosphorus in X-epi-CD2. the charge transfers (QNBO and QMulliken) between epi-Cinchonidine and an analyte present less deviation in X-epi-CD1 (up to 0.117 and 0.311 e−) than the values in X-epi-CD2 (up to 0.467 and 0.179 e−). Even though electron-withdrawing substituents tend to present the enhanced charge transfer, the highly steric hindered nitro group showed bigger QNBO and QMulliken in X-epi-CD2 (N16) than in X-epi-CD1 (O36). In mono-substituted phosphine, XPH2, σ*(XP) was the LUMO that withdrew the electrons from the HOMO of N16 of quinoline or hydroxyl (−O36H37) at C17.
In this study, at B3LYP/6-31G(d) and B3LYPgCP-D3/6-31G levels of theory, intermolecular interactions of epi-Cinchonidine with analytes (X) were described by the geometrical parameters and electronic, thermodynamic, and charge analyses. O36 of the hydroxyl group (in X-epi-CD1) and N16 of the quinoline ring (in X-epi-CD2) among Lewis basic atoms of epi-Cinchonidine could be the interacting atoms of pnicogen bonds. While the dominant force of hydrogen bonding generally is electrostatic attraction, HOMO–LUMO energy, QNBO, QMulliken, AIM analysis, and UV-Vis analysis of the pnicogen bonds elucidated the interaction including the polarization and electron transfer. Based on the results, researchers can further progress pnicogen-based asymmetric catalysis based on our study in the recent future.
Bioinspired Synthesis of (+)-Cinchonidine Using Cascade Reactions
The development of efficient syntheses of complex natural products has long been a major challenge in synthetic chemistry. Designing cascade reactions and employing bioinspired transformations are an important and reliable means of achieving this goal. Presented here is a combination of these two strategies, which allow efficient asymmetric synthesis of the cinchona alkaloid (+)-cinchonidine. The key steps of this synthesis are a controllable, visible-light-induced photoredox radical cascade reaction to efficiently access the tetracyclic monoterpenoid indole alkaloid core, as well as a practical biomimetic cascade rearrangement for the indole to quinoline transformation. The use of stereoselective chemical transformations in this work makes it an efficient synthesis of (+)-cinchonidine.[3]
Herein we present an efficient asymmetric approach to (+)-cinchonidine [(+)-3] and it features two key cascade reactions, namely, a controllable, photoinduced radical cascade cyclization and a biomimetic cascade rearrangement. Consequently, treatment with aqueous NaOH in isopropanol at 50 °C resulted in the hydrolysis of NCbz group and subsequent intramolecular N4 alkylation, thus yielding (+)-cinchonidine [[(+)-3]]. The spectra and physical properties of the synthetic sample matched those of the natural (−)-cinchonidine [(−)-3], except for the sign of the optical rotation [synthetic: [α]D20=+91.8 (c=0.17, EtOH); natural: [α]D20=−95.9 (c=0.27, EtOH)].
In summary, we have achieved a concise approach to the cinchona alkaloid (+)-cinchonidine. The efficacy of our synthesis relies on a controllable, scalable, visible-light-induced photoredox radical cascade reaction to generate the tetracyclic monoterpenoid indole alkaloid core and on the development of a practical biomimetic cascade rearrangement to enable the indole to quinoline transformation. Of additional note is the excellent chemo-, regio-, and stereoselectivity achieved in this synthetic route, which greatly contributes to the synthesis of the historically important and useful cinchona alkaloids.
References
[1]Mishra N, Agrahari AK, Bose P, Singh SK, Singh AS, Tiwari VK. Click Inspired Synthesis of Novel Cinchonidine Glycoconjugates as Promising Plasmepsin Inhibitors. Sci Rep. 2020 Feb 27;10(1):3586.
[2]Ullah Z, Kim K, Venkanna A, Kim HS, Kim MI, Kim MH. Plausible Pnicogen Bonding of epi-Cinchonidine as a Chiral Scaffold in Catalysis. Front Chem. 2021 Jul 6;9:669515.
[3]Liu, Wentao et al. “Bioinspired Synthesis of (+)-Cinchonidine Using Cascade Reactions.” Angewandte Chemie (International ed. in English) vol. 57,38 (2018): 12299-12302.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringIodixanol is an iso-osmolal nonionic dimeric hydrophilic contrast agent. It has similar diagnostic efficacy to that of other iodinated contrast media.....
Apr 24,2025DrugsCinchonidine
485-71-2You may like
- Cinchonidine
-
- $44.00 / 5g
- 2025-12-05
- CAS:485-71-2
- Min. Order:
- Purity: 98.25%
- Supply Ability: 10g
- Cinchonidine
-
- $44.00 / 5g
- 2025-12-05
- CAS:485-71-2
- Min. Order:
- Purity: 98.25%
- Supply Ability: 10g
- Cinchonidine
-

- $371.00 / 1KG
- 2025-12-03
- CAS:485-71-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100kg