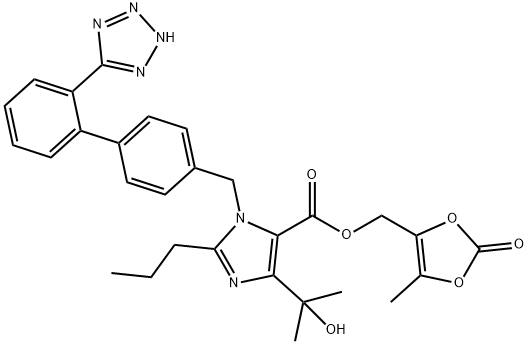
Efficacy and safety of Olmesartan Medoxomil in the treatment of hypertension
Jan 5,2024
Description of Olmesartan Medoxomil
Olmesartan medoxomil is an angiotensin II type 1 (AT(1)) receptor antagonist (angiotensin receptor blocker [ARB]) that inhibits the actions of angiotensin II on the renin-angiotensin-aldosterone system, which plays a key role in the pathogenesis of hypertension. Oral olmesartan medoxomil 10-40 mg once daily is recommended for the treatment of adult patients with hypertension. Fixed-dose combination tablets of other drugs (olmesartan medoxomil, amlodipine and hydrochlorothiazide (HCTZ)) may be used in combination with other drugs in patients with poorly controlled blood pressure on monotherapy.
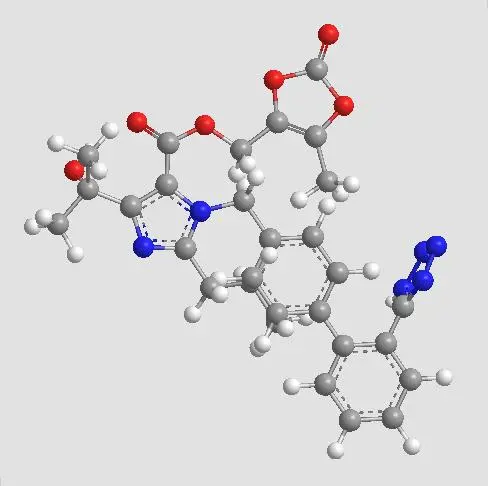
Efficacy and safety of Olmesartan Medoxomil
Olmesartan medoxomil has been reported to be an effective agent for the treatment of hypertension. Its blood pressure-lowering effects were comparable to those of other antihypertensive agents and other ARBs. Effects were seen as early as 2 weeks and persisted when olmesartan medoxomil was administered long term (for 1 year). The maximum recommended daily dose is 40 mg, except in the presence of severe renal insufficiency (creatinine clearance<20 mL/min) or moderate hepatic insufficiency (Child-Pugh score 7–9), when the daily dose should not exceed 20 mg. Olmesartan medoxomil was well tolerated. The most commonly reported adverse effect occurring significantly more often with olmesartan medoxomil than with placebo was dizziness (seen in ∼3% of patients). The occurrence of clinically significant drug interactions was minimal.
Olmesartan medoxomil is an orally administered angiotensin II receptor antagonist, selective for the angiotensin II type 1 receptor, which has established antihypertensive efficacy in adults. In children and adolescents with hypertension (n = 302), oral olmesartan medoxomil significantly and dose-dependently reduced seated systolic blood pressure (BP) and seated dystolic BP from baseline (the primary endpoint) in a 3-week, dose-response period in a well designed phase II/III clinical trial. Patients received olmesartan medoxomil high dose (20 or 40 mg once daily depending on bodyweight) or low dose (2.5 or 5.0 mg once daily depending on bodyweight). The response was significant for both cohorts, which were stratified by race (cohort A was mixed race [62% White] and cohort B was 100% Black). In addition, BP control was maintained in olmesartan recipients relative to placebo recipients in cohort A and the combined cohort A + B, but not for patients in cohort B, during a placebo-controlled withdrawal period of this trial. Oral olmesartan medoxomil was generally well tolerated in children and adolescents with hypertension. The majority of adverse events were of mild to moderate intensity.
The antihypertensive agents olmesartan medoxomil, amlodipine and hydrochlorothiazide (HCTZ) are now available as a fixed-dose combination tablet (olmesartan medoxomil/amlodipine/HCTZ). In a 12-week, randomized, double-blind, multicentre trial (TRINITY) in adults with moderate to severe hypertension, olmesartan medoxomil + amlodipine + HCTZ triple combination therapy produced significantly greater least squares mean reductions from baseline in seated diastolic blood pressure (BP) [primary endpoint] and seated systolic BP than olmesartan medoxomil/amlodipine, olmesartan medoxomil/HCTZ or amlodipine + HCTZ. Furthermore, significantly more patients achieved BP goals and targets with the triple combination regimen than with any of the dual combination regimens at week 12, with olmesartan medoxomil + amlodipine + HCTZ also demonstrating benefit over the dual regimens in terms of ambulatory BP control. According to subgroup analyses of the TRINITY trial, olmesartan medoxomil + amlodipine + HCTZ was more effective in reducing BP and achieving BP goals than each of the dual therapies, irrespective of hypertension severity, age, sex, race or diabetes mellitus status. Data from a number of smaller clinical studies indicated that olmesartan medoxomil + amlodipine + HCTZ triple combination therapy provides antihypertensive efficacy in patients whose BP is not adequately controlled with olmesartan medoxomil + amlodipine. Olmesartan medoxomil + amlodipine + HCTZ was generally well tolerated in the TRINITY study, with adverse events usually being mild or moderate in severity.
References:
[1] PHARMD J A B, BCPS; John M B P, FASHP, et al. Olmesartan medoxomil: An angiotensin II-receptor blocker[J]. Clinical therapeutics, 2003. DOI:10.1016/S0149-2918(03)80066-8.
[2] VICTORIA J MUIR; Gillian M K. Olmesartan medoxomil: in children and adolescents with hypertension.[J]. Drugs, 2010. DOI:10.2165/11206310-000000000-00000.
[3] DEEKS E D. Olmesartan medoxomil/amlodipine/hydrochlorothiazide: fixed-dose combination in hypertension.[J]. Drugs, 2011. DOI:10.2165/11206770-000000000-00000.
- Related articles
- Related Qustion
- Olmesartan Medoxomil: Pharmacokinetics and Clinical Applications Mar 6, 2024
Olmesartan medoxomil, rapidly converted to olmesartan, is a common angiotensin II receptor blocker effective in treating hypertension, heart failure, and diabetic nephropathy.
- Olmesartan medoxomil: pharmacological properties, therapeutic efficacy and tolerability Sep 27, 2023
Olmesartan medoxomil is an effective and safe ARB that lowers blood pressure, improves arterial compliance, and protects against end-organ damage.
- What is Olmesartan medoxomil?Uses_Side Effects_Usuage Sep 18, 2020
Olmesartan medoxomil is a white to light yellowish-white powder or crystalline powder with a molecular weight of 558.59. It is practically insoluble in water and sparingly soluble in methanol.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical Engineering2,5-Furandicarboxylic acid can replace petroleum-based terephthalic acid to produce biobased plastic polyethylene furan dicarboxylate (PEF) due to the similarity in its functional group with terephthalic acid.....
Jan 5,2024Organic ChemistryOlmesartan Medoxomil
144689-63-4You may like
- Clinical Application Research of Sertraline
Dec 19, 2025
- Nandrolone: brief history and application research
Dec 18, 2025
- Application research of Ceftiofur hydrochloride
Dec 18, 2025
Olmesartan Medoxomil manufacturers
- Olmesartan Medoxomil
-

- $0.00 / 1KG
- 2025-12-23
- CAS:144689-63-4
- Min. Order: 1KG
- Purity: 99.0%,USP/EP
- Supply Ability: 500KG/month
- Olmesartan Medoxomil
-

- $30.00 / 5mg
- 2025-12-23
- CAS:144689-63-4
- Min. Order:
- Purity: 99.90%
- Supply Ability: 10g
- Olmesartan medoxomil
-
- 2025-12-23
- CAS:144689-63-4
- Min. Order:
- Purity: 0.99
- Supply Ability: