Ethyl-2,2-dimethyl-7-bromoheptanoate: Applications as Pharmaceutical Intermediate and Preparation Method
Apr 8,2024
General Description
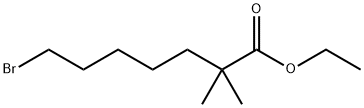
Ethyl-2,2-dimethyl-7-bromoheptanoate is a valuable organic compound with unique structural features, including an ethyl ester group linked to a heptanoic acid chain with a bromine atom at the 7-position. Its chemical properties make it versatile for various synthetic applications. Specifically, Ethyl-2,2-dimethyl-7-bromoheptanoate serves as a crucial intermediate in the synthesis of bempedoic acid, a treatment for hypercholesterolemia and cardiovascular disease. Ethyl-2,2-dimethyl-7-bromoheptanoate's efficiency in alkylation reactions leads to high yields and purity, ensuring safety, cost-effectiveness, and suitability for large-scale production. The preparation method involves specific starting materials and conditions, utilizing sodium hydride instead of lithium reagents for improved safety and manageability. This innovative approach streamlines synthesis, addressing challenges associated with traditional methods. In conclusion, ethyl-2,2-dimethyl-7-bromoheptanoate plays a pivotal role in pharmaceutical applications and represents a significant advancement in organic synthesis methodologies.

Figure 1. Ethyl-2,2-dimethyl-7-bromoheptanoate
Overview
Ethyl-2,2-dimethyl-7-bromoheptanoate is a significant organic compound known for its distinct structural composition, comprising an ethyl ester group connected to a heptanoic acid chain with a bromine atom at the 7-position. This unique configuration imparts specific chemical properties to the compound, enhancing its utility in diverse synthetic processes. The presence of two methyl groups on the second carbon of the heptanoic acid chain further contributes to its stability and reactivity. Overall, ethyl-2,2-dimethyl-7-bromoheptanoate serves as a valuable intermediate in organic synthesis, offering a broad spectrum of potential applications within the chemical industry. Its versatile nature and characteristic features position it as a key component in various synthetic pathways, highlighting its importance in driving innovation and advancements in chemical research and manufacturing. 1
Applications as Pharmaceutical Intermediate
Ethyl-2,2-dimethyl-7-bromoheptanoate plays a crucial role as an intermediate in the preparation of bempedoic acid, which is utilized for treating hypercholesterolemia and atherosclerotic cardiovascular disease. Ethyl-2,2-dimethyl-7-bromoheptanoate serves as a key building block in the synthesis of bempedoic acid intermediates, facilitating a streamlined and efficient manufacturing process. Specifically, in the context of this application, Ethyl-2,2-dimethyl-7-bromoheptanoate (R=Et) is employed in the alkylation reaction with 1-[(isocyanomethyl)sulfonyl]-4-methyl-benzene to produce the desired compound I. This alkylation process results in a remarkable yield of 94.2%, exemplifying the high efficiency and effectiveness of this intermediate in the overall synthetic pathway. The utilization of Ethyl-2,2-dimethyl-7-bromoheptanoate in this context offers several significant advantages. Firstly, it contributes to the high safety standards of the overall process, ensuring a reliable and secure production environment. Secondly, it offers cost benefits, contributing to the economic viability of the synthesis of bempedoic acid. Additionally, the high yield and purity of the resulting intermediates further enhance its suitability for large-scale industrial production. Overall, the application of Ethyl-2,2-dimethyl-7-bromoheptanoate as an intermediate in the preparation of bempedoic acid highlights its pivotal role in the pharmaceutical industry, specifically in the development of treatments for hypercholesterolemia and atherosclerotic cardiovascular disease. 2
Preparation Method
Preparation of Ethyl-2,2-dimethyl-7-bromoheptanoate involves a straightforward and efficient method using specific starting materials and conditions. The process begins with the utilization of ethyl isobutyrate, 1,5-dibromopentane, and sodium hydride as key components. These substances are then subjected to a reaction in N,N-dimethylpropenylurea under controlled conditions. The reaction takes place within a temperature range of 5-85°C over a duration of 20-25 hours. Through this controlled environment, Ethyl-2,2-dimethyl-7-bromoheptanoate is successfully synthesized. This method offers distinct advantages over traditional approaches by circumventing the challenges associated with using lithium reagents, known for their unpredictable reactivity. Instead of lithium reagents, sodium hydride serves as the strong base, ensuring a safer and more manageable reaction process. By utilizing this innovative method, the synthesis becomes streamlined, facilitating ease of operation and scalability for industrial production. Moreover, this approach addresses concerns related to the safety and cost implications of employing lithium reagents in large-scale manufacturing settings. Overall, the preparation method for Ethyl-2,2-dimethyl-7-bromoheptanoate represents a significant advancement in the field, offering a practical and efficient solution to existing challenges. 3
Reference
1. Ethyl 7-Bromo-2,2-dimethylheptanoate. National Center for Biotechnology Information. 2024; PubChem Compound Summary for CID 11265571.
2. Zhang X, Rao X, Ning DB. Preparation method of bempedoic acid intermediates, and preparation method of bempedoic acid for treating hypercholesterolemia and atherosclerotic cardiovascular disease. 2022; Patent Number: CN115504914.
3. Gao Y, Chen DD, Li ZX. Preparation of ethyl 7-bromo-2,2-dimethyl heptanoate. 2020; Patent Number: CN112047840.
- Related articles
- Related Qustion
- Ethyl-2,2-dimethyl-7-bromoheptanoate:Synthesis and Application Dec 8, 2022
Ethyl-2,2-dimethyl-7-bromoheptanoate is an important raw material for the synthesis of bempedoic acid.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringGinkgolide B from Ginkgo biloba offers neuroprotection by reducing infarct size, enhancing survival, and modulating cellular pathways in ischemic strokes.....
Nov 11,2024Natural Productsethyl-2,2-dimethyl-7-bromoheptanoate
123469-92-1You may like
ethyl-2,2-dimethyl-7-bromoheptanoate manufacturers
- ethyl-2,2-dimethyl-7-bromoheptanoate
-
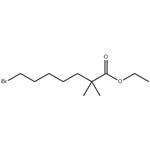
- $8.00 / 1kg
- 2025-09-25
- CAS:123469-92-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- Ethyl-2,2-dimethyl-7-bromoheptanoate
-

- $0.00 / 1KG
- 2024-05-20
- CAS:123469-92-1
- Min. Order: 1KG
- Purity: 97.0-99.0%min GC
- Supply Ability: 5-10tons/year
- ethyl-2,2-dimethyl-7-bromoheptanoate
-
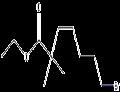
- $0.01 / 1KG
- 2019-12-25
- CAS:123469-92-1
- Min. Order: 1KG
- Purity: 95%-99%
- Supply Ability: 1kg; 100kg; 500kg