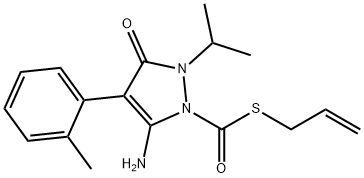
Fenpyrazamine: Synthesis and Introduction
Feb 4,2024
Synthesis of Fenpyrazamine
The synthesis of fenpyrazamine starts with methyl 2-cyano-2-(o-tolyl)acetate. The specific synthesis steps are as follows:
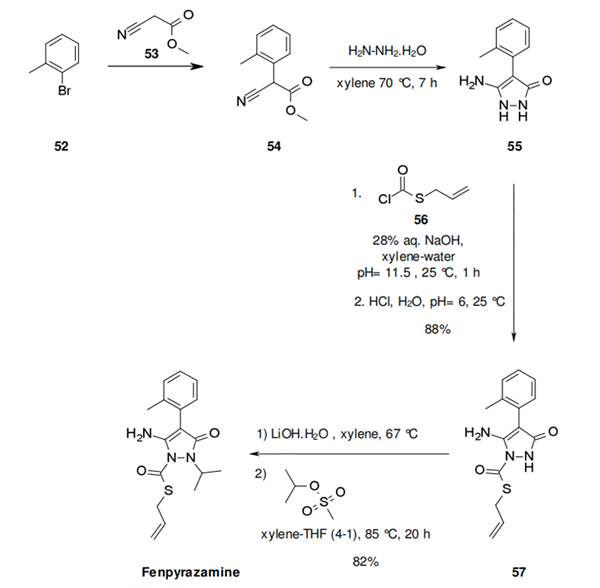
The synthesis of fenpyrazamine starts with methyl 2-cyano-2-(o-tolyl)acetate (54). It has been reported in the literature that compounds 54 can be formed from the coupling of the bromotolyl 52 or the corresponding chloride and the cyano-acetate 53 by a metal catalysed coupling process. 60 The formation of the 5-amino-1,2-dihydropyrazol-3-one (55) is performed by condensation of hydrazine hydrate with the cyano acetate 54, by an azeotropic distillation of water, followed by condensation. The yield for this reaction has not been reported, but there is no doubt that this is high yielding reaction. S-allyl chloromethanethioate (56) is introduced with high regioselectively, as shown in scheme 8, yielding compound 57, although there are potentially 2 other reactive nitrogens. The choice of base and the mixture of water and xylene may play a major role in this selectivity. Finally, the addition of isopropyl methane sulphonate in the presence of lithium hydroxide provides fenpyrazamine.
The S-allyl chloromethanethioate 56 is prepared from the reaction of allyl mercaptan with phosgene in the presence of a catalytic amount of triethylamine in excellent yield [S-allylmercaptan, phosgene, cat NEt3, xylene, 40 °C, 11 h]. All the reactions described are performed in xylene, suggesting good opportunities for telescoping in manufacturing and reducing costs.
Introduction of Fenpyrazamine
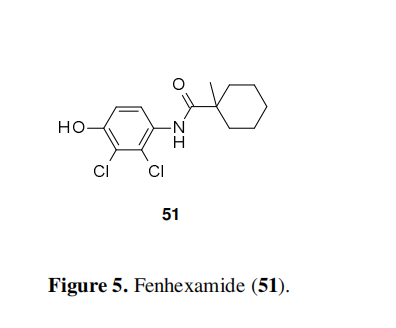
Fenpyrazamine is a fungicide that was discovered and developed by Sumitomo Chemical Company. The molecule is particularly efficient against the Sclerotiniaceae family such as B. cinerea (grey mould), other Botrytis spp. and Sclerotinia spp. Fenpyrazamine exerts its antifungal activity by inhibiting the sterol biosynthesis, more particularly the 3-keto reductase of the enzymatic complex of the sterol C-4 demethylation. This is the second molecule, after fenhexamide 51 (Figure 5), reaching the crop protection market with this particular mode of action. The two molecules have completely different scaffolds. It is not yet known if the molecules are acting on the same active site or not.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringLithium Borohydride is a white solid with multiple crystal forms, soluble in polar solvents. Research aims to enhance its hydrogen storage properties for practical applications.....
Feb 4,2024APIFenpyrazamine
473798-59-3You may like
- fenpyrazamine
-

- $0.00 / 25kg
- 2025-12-02
- CAS:473798-59-3
- Min. Order: 1kg
- Purity: 99
- Supply Ability: 20tons
- 5-Amino-2,3-dihydro-2-(1-methylethyl)-4-(2-methylphenyl)-3-oxo-1H-pyrazole-1-carbothioic acid S-2-propen-1-yl ester
-

- $1.00 / 1KG
- 2019-07-06
- CAS:473798-59-3
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 1ton