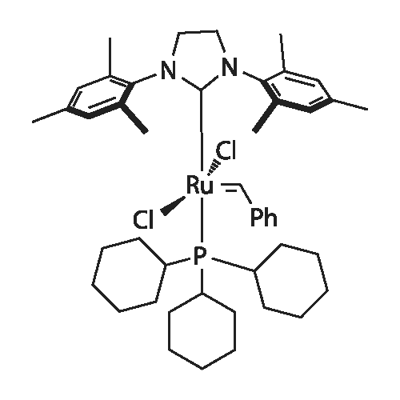
Grubbs catalyst 2nd generation: Ruthenium-Based Olefin Metathesis Catalyst
Dec 18,2025
The Grubbs Catalyst 2nd Generation is a highly effective ruthenium-based catalyst used primarily for olefin metathesis, offering improved stability and reactivity compared to its predecessor. The 2nd Generation Grubbs Catalyst features a ligand exchange where one of the tricyclohexylphosphine ligands is replaced with a more stable N-heterocyclic carbene (NHC) ligand. The Grubbs catalyst 2nd generation demonstrates superior thermal and chemical stability compared to the first generation, enabling its use under a broader range of reaction conditions. It efficiently promotes ring-closing metathesis (RCM), ring-opening metathesis polymerization (ROMP), and cross-metathesis (CM) reactions, offering high yields and excellent functional group tolerance

Merging Grubbs catalyst 2nd generation with photocatalysis
Olefin cross-metathesis is undoubtedly one of the most powerful methodologies for the formation of carbon–carbon double bonds. Thanks to the rational development of robust metathesis catalysts, this chemical transformation is now involved in several branches of science, including synthetic organic chemistry, material science, and biochemistry. While bench-stable and easily accessible metathesis catalysts such as the well-known Grubbs catalyst 2nd generation and Grubbs–Hoveyda catalysts enabled the exclusive formation of the thermodynamically favored E-olefins, access to the contra-thermodynamic Z-isomers required the use of sophisticated transition metal catalysts. To test our hypothesis, we first selected homodimerization of styrene 7a in the presence of the Grubbs second-generation catalyst 2 with different photocatalysts. The choice of the Grubbs catalyst 2nd generation was motivated by a recent study by Erasmus et al., who investigated the electrochemistry of this catalyst and showed that it follows an EC mechanism, where the first oxidation step is fast and reversible followed by a slow chemical step.[1]
We first examined the interaction of blue light with Grubbs catalyst 2nd generation to confirm that light was not deleterious to the metathesis process. In this context, several DFT calculations were performed, starting with inspecting the optimized ground state molecular orbitals and analyzing the TD-DFT calculation of Franck–Condon excited states. We have developed an orthogonal tandem catalytic (metathesis/photoisomerization) transformation enabling facile access to Z-olefins from the standard E-selective Grubbs catalyst 2nd generation. Joint experimental and computational investigations showed that the feasibility of this reaction results from a combination of (i) light being mostly absorbed by the photocatalyst PC, (ii) diffusion-controlled back electron transfer restoring Grubbs catalyst following its oxidation by PC* (iii) formation of the classical metathesis product E-alkene, and (iv) E–Z photoisomerization by a final energy transfer event from PC.
Electrochemical Properties of Grubbs catalyst 2nd generation
Since the first reported Grubbs metathesis catalyst in 1992, the field of ruthenium-based carbon–carbon bond formation reactions via metathesis methodology has gained considerable interest and continues to do so with its vast array of applications in cross-metathesis, self-metathesis, acyclic diene metathesis polymerization, ring-opening metathesis, ring-opening metathesis polymerization, ring-closing metathesis, asymmetric ring-closing metathesis, and ene–yne metathesis. Testimony to this is the use of Grubbs catalyst 2nd generation as the catalysts of choice in the preparation of fine chemicals, biologically active chemicals and drug fragments, industrial applications, polymer synthesis, and polymer recycling. However, no comparative electronic study (UV–vis, electrochemical) on the valence and core electrons of Grubbs catalyst 2nd generationcould be found. A few articles on the electrochemical behavior of ruthenium carbene complexes with structures similar to those of Grubbs’ catalysts report a one-electron RuII/RuIII oxidation that may be reversible or irreversible, depending on the compound.[2]
According to UV–vis spectroscopy (0.10 mM, CH2Cl2 at 25 °C), the catalyst transformation (which could possibly include ligand dissociation with active catalyst formation, dimer formation, and decomposition) rate constants (kobs) of Grubbs catalyst 2nd generation is 7.48 × 10–5. In the case of Grubbs catalyst 2nd generation, a second reduction peak appeared at slow scan rates. This may probably be ascribed to an electrochemically active compound that was formed from the intermediate cation and the subsequent reduction of the latter.
References
[1]Chérif SE, Ghosh A, Chelli S, Dixon IM, Kraiem J, Lakhdar S. Merging Grubbs second-generation catalyst with photocatalysis enables Z-selective metathesis of olefins: scope, limitations, and mechanism. Chem Sci. 2022 Sep 22;13(41):12065-12070. doi: 10.1039/d2sc03961c. PMID: 36349104; PMCID: PMC9600307.
[2]Swart MR, Marais C, Erasmus E. Comparison of the Spectroscopically Measured Catalyst Transformation and Electrochemical Properties of Grubbs' First- and Second-Generation Catalysts. ACS Omega. 2021 Oct 21;6(43):28642-28653. doi: 10.1021/acsomega.1c03109. PMID: 34746559; PMCID: PMC8567268.
- Related articles
- Related Qustion
Vardenafil is a potent and highly selective inhibitor of PDE-5. Relevant information were introduced, such as pharmacodynamic properties, mechanism.....
Dec 18,2025DrugsCeftiofur exhibits a good activity against a broad range of gram-negative and gram-positive bacteria, including many that produce β-lactamase.....
Dec 18,2025DrugsGrubbs catalyst 2nd generation
246047-72-3You may like
Grubbs catalyst 2nd generation manufacturers
- Grubbs catalyst 2nd generation
-

- $10.00 / 1ASSAYS
- 2025-12-18
- CAS:246047-72-3
- Min. Order: 1ASSAYS
- Purity: 99%
- Supply Ability: 1 ton
- Grubbs catalyst 2nd generation
-
- $132.00 / 5g
- 2025-12-18
- CAS:246047-72-3
- Min. Order: 5g
- Purity: 0.98
- Supply Ability: 10kg
- Grubbs Catalyst 2nd Generation
-

- $0.00 / 25KG
- 2025-12-18
- CAS:246047-72-3
- Min. Order: 1KG
- Purity: ≥99%
- Supply Ability: 100mt/year