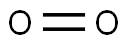Oxygen:Sources,electronic configuration,uses,production
May 27,2024
Oxygen is the most abundant chemical element by mass in the Earth's biosphere, air, sea, and land (Box 3.8). Oxygen constitutes 49.2% of the Earth's crust by mass as part of oxide compounds such as silicon dioxide, and SiO2, and is the most abundant element by mass in the Earth's crust.
Sources
Oxygen (besides H) is the most common element in minerals and forms the basis of the anionic groups (carbonates, nitrates, borates, sulfates, chromates, phosphates, arsenates, vanadates, molybdates, tungstates, silicates, etc.). The chemically simplest minerals are found in the oxide/hydroxide class, for example, anatase (TiO2), bixbyite (Mn2O3), bo¨hmite (AlOOH), brookite (TiO2), etc.

Fig.quartz
Chemistry
Oxygen is a member of the chalcogen group on the periodic table. The electronic configuration of the free O atom is [He]2s22p2x2p1y2p1z. Oxygen is normally divalent, though, other oxidation states such as 1/2, 0, in isolable compounds are also known. It has a very high electronegativity of 3.5, which is exceeded.
Production
Oxygen gas can also be produced through the electrolysis of water into molecular oxygen and hydrogen. DC electricity must be used: if AC is used, the gases in each limb consist of hydrogen and oxygen in the explosive ratio of 2:1.
Uses
Oxygen is used in cellular respiration and many important groups of organic molecules in living organisms including humans contain oxygen, for example, proteins, nucleic acids, carbohydrates, and fats, as do the most important constituent inorganic compounds of animal shells, teeth, and bone (calcite, aragonite, apatite, etc.). Most of the mass of living organisms contains oxygen as a part of water, H2O.
- Related articles
- Related Qustion
- what is the atomic number of the element located in group 16, period 2 of the periodic table? Mar 7, 2024
The atomic number of the element in Group 16, Period 2 of the Periodic Table is 8 and the element is oxygen.
- What is oxygen used for? Is it an element? Mar 4, 2024
Oxygen forms a molecule (O2) of two atoms which is a colorless gas at normal temperatures and pressures.Oxygen is the most abundant element in Earth's crust, and after hydrogen and helium.
- How to determine the polarity of oxygen? Dec 20, 2023
Oxygen is an oxidizing agent that forms oxides with multiple compounds readily. There are many known allotropes of oxygen, out of which O2 is the most stable one.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringNumerous silicate minerals have direct commercial applications, for example, clays, silica sand, and most kinds of building stone. Hence, most uses for silicon are as structural compounds, either as the silicate minerals or silica.....
May 27,2024Inorganic chemistryOxygen
7782-44-7You may like
- Oxygen USP/EP/BP
-
- $1.10 / 1g
- 2025-11-18
- CAS:7782-44-7
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons min