Paclitaxel: Medical use, mechanism, side effects
May 11,2023
General description
Paclitaxel (PTX), sold under the brand name Taxol among others, is a chemotherapy medication used to treat a number of types of cancer.[1] This includes ovarian cancer, esophageal cancer, breast cancer, lung cancer, Kaposi sarcoma, cervical cancer, and pancreatic cancer. It is given by injection into a vein. There is also an albumin-bound formulation. Paclitaxel was first isolated in 1971 from the Pacific yew and approved for medical use in 1993. It is on the World Health Organization's List of Essential Medicines. It has been made from precursors, and more recently through cell culture. Its appearance is as follows:

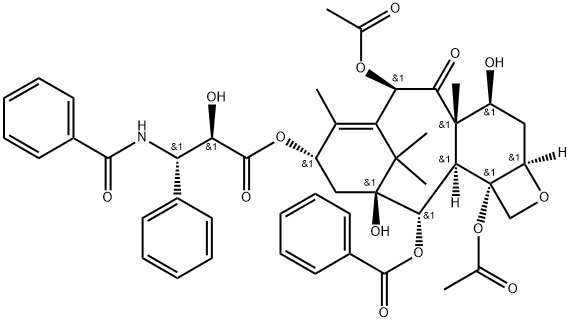
Figure 1 The appearance of Paclitaxel
Medical use
Paclitaxel is approved in the UK for ovarian, breast, lung, bladder, prostate, melanoma, esophageal, and other types of solid tumor cancers as well as Kaposi's sarcoma.[2] It is recommended in National Institute for Health and Care Excellence (NICE) guidance of June 2001 that it should be used for non-small-cell lung cancer in patients unsuitable for curative treatment, and in first-line and second-line treatment of ovarian cancer. In September 2001, NICE recommended paclitaxel should be available for the treatment of advanced breast cancer after the failure of anthracyclic chemotherapy, but that its first-line use should be limited to clinical trials. In September 2006, NICE recommended paclitaxel should not be used in the adjuvant treatment of early node-positive breast cancer. In 2018, it is approved in the United States for the treatment of breast, pancreatic, ovarian, Kaposi's sarcoma and non-small-cell lung cancers. Albumin-bound paclitaxel (trade name Abraxane, also called nab-paclitaxel) is an alternative formulation where paclitaxel is bound to albumin nanoparticles. Much of the clinical toxicity of paclitaxel is associated with the solvent Cremophor EL in which it is dissolved for delivery.[3]
Paclitaxel is used as an antiproliferative agent for the prevention of restenosis (recurrent narrowing) of coronary and peripheral stents; locally delivered to the wall of the artery, a paclitaxel coating limits the growth of neointima (scar tissue) within stents.[4] Paclitaxel drug-eluting stents for coronary artery placement are sold under the trade name Taxus by Boston Scientific in the United States. Paclitaxel drug-eluting stents for femoropopliteal artery placement are also available.
Mechanism
Paclitaxel is one of several cytoskeletal drugs that target tubulin. Paclitaxel-treated cells have defects in mitotic spindle assembly, chromosome segregation, and cell division. Unlike other tubulin-targeting drugs, such as colchicine, that inhibit microtubule assembly, paclitaxel stabilizes the microtubule polymer and protects it from disassembly. Chromosomes are thus unable to achieve a metaphase spindle configuration. This blocks the progression of mitosis and prolonged activation of the mitotic checkpoint triggers apoptosis or reversion to the G0-phase of the cell cycle without cell division.[5] The ability of paclitaxel to inhibit spindle function is generally attributed to its suppression of microtubule dynamics, but other studies have demonstrated that suppression of dynamics occurs at concentrations lower than those needed to block mitosis. At the higher therapeutic concentrations, paclitaxel appears to suppress microtubule detachment from centrosomes, a process normally activated during mitosis. Paclitaxel binds to the beta-tubulin subunits of microtubules.
Side effects
Common side effects include nausea and vomiting, loss of appetite, change in taste, thinned or brittle hair, pain in the joints of the arms or legs lasting two to three days, changes in the color of the nails, and tingling in the hands or toes. More serious side effects such as unusual bruising or bleeding, pain, redness or swelling at the injection site, hand-foot syndrome, change in normal bowel habits for more than two days, fever, chills, cough, sore throat, difficulty swallowing, dizziness, shortness of breath, severe exhaustion, skin rash, facial flushing, female infertility by ovarian damage, and chest pain can also occur. Neuropathy may also occur.[1] A number of these side effects are associated with the excipient used, Cremophor EL, a polyoxyethylated castor oil, and allergies to cyclosporine, teniposide, and other drugs containing polyoxyethylated castor oil may increase the risk of adverse reactions to paclitaxel.
References
[1]Paclitaxel. The American Society of Health-System Pharmacists. Archived from the original on September 14, 2017. Retrieved January 2, 2015.
[2]Saville MW, Lietzau J, Pluda JM, Feuerstein I, Odom J, Wilson WH, et al. (July 1995). Treatment of HIV-associated Kaposi's sarcoma with paclitaxel. Lancet (Submitted manuscript). 346 (8966): 26–8.
[3]Gelderblom H, Verweij J, Nooter K, Sparreboom A (September 2001). Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. European Journal of Cancer. 37 (13): 1590–8.
[4]Heldman AW, Cheng L, Jenkins GM, Heller PF, Kim DW, Ware M, et al. (May 2001). Paclitaxel stent coating inhibits neointimal hyperplasia at 4 weeks in a porcine model of coronary restenosis. Circulation. 103 (18): 2289–95.
[5]Brito DA, Yang Z, Rieder CL (August 2008). Microtubules do not promote mitotic slippage when the spindle assembly checkpoint cannot be satisfied. The Journal of Cell Biology. 182 (4): 623–9.
- Related articles
- Related Qustion
- Adverse Reactions and Precautions of Paclitaxel Jun 23, 2025
Paclitaxel, as a third-generation chemotherapy drug, is a landmark tumor chemotherapy drug. It has dealt with many cancer cells, and its efficacy has been verified by long-term clinical practice.
- Does nanodispersion-based paclitaxel injection have better antitumor effects than paclitaxel? Dec 16, 2024
Yes. Currently, researchers have developed a nanoparticle-based concentrate for injection (Nab-paclitaxel), which is a water-soluble formulation of paclitaxel.
- What is Paclitaxel used for? Aug 20, 2024
Paclitaxel is a widely used drug developed since the mid-1980s, with significant antitumor activity against ovarian, head and neck, bladder, breast and lung cancers.
Paclitaxel
33069-62-4You may like
- TAXOL C(P)
-

- $5.00/ KG
- 2025-12-15
- CAS:33069-62-4
- Min. Order: 1KG
- Purity: 99% hplc
- Supply Ability: 500TONS
- Paclitaxel
-

- $5.00/ KG
- 2025-12-15
- CAS:33069-62-4
- Min. Order: 0.10000000149011612KG
- Purity: 99% hplc
- Supply Ability: 5000kg
- Paclitaxel
-

- $0.00 / 1g
- 2025-12-15
- CAS:33069-62-4
- Min. Order: 1g
- Purity: 97%-102%
- Supply Ability: 1000g