Pharmacology research of Genipin
Oct 15,2025
Introduction
Genipin (GNP), an iridoid derivative found in the fruits of Genipa americana and Gardenia jasminoides, has gained significant attention as a naturally occurring cross-linking polymer. Genipin is biodegradable because it has 10-fold less cytotoxicity than glutaraldehyde. Genipin exhibits exceptional biocompatibility, allowing for direct introduction into living tissue. This particular advantage is further highlighted by its significantly lower cytotoxicity when compared to conventional and synthetic cross-linkers like glutaraldehyde and epoxy compounds. Genipin can react with primary amines in proteins or polysaccharides, making it suitable for crosslinking. In addition to cross-linking, genipin has been widely utilized for polysaccharide and protein-polysaccharide conjugation, resulting in improved properties such as enhanced emulsifying abilities and overall physical properties. Moreover, genipin has been confirmed to possess medicinal benefits, including anti-inflammatory, antibacterial, antioxidative, antithrombotic, hepatoprotective, and choleretic effects. Because of these advantages, genipin is an important cross-linking agent due to its tunable properties and versatile applications such as food,medical, and pharmaceutical. Genipin is extracted from the Gardenia (Gardenia jasminoides J.Ellis) and the Genipa americana L. It has been shown that both plants, an essential part of the family Rubiaceae, can be considered a potential precursor of natural blue pigment for food products.[1]
Physicochemical characteristics of genipin
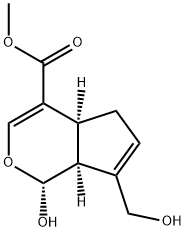
Genipin possesses distinct physicochemical characteristics. Genipin is a water-soluble white crystalline powder.(Figure 1) It contains a dihydropyran ring and an ester group and a molecular weight of 226 mg/g. Genipin is C11H14O5, or methyl-1-hydroxy-7-(hydroxymethyl)-1,4a,5,7a-tetrahydrocyclopenta[c]pyran-4-carboxylate. It can be soluble in various solvents, including alcohol (methanol or ethanol), saline, water, propylene glycol, ethyl acetate, and acetone. A recent study has found that Genipin’s biocompatibility and low toxicity make it an excellent choice for biomedical applications and controlled drug release. Genipin has been identified as the primary active compound through pharmacokinetic studies, demonstrating potential as a potent neuroprotective agent by effectively inhibiting high lactate dehydrogenase (LDH) levels in the bloodstream.This inhibition helps prevent the toxic effects of amyloid-β (Aβ) peptides on cultured neuronal cells. It has been found that piperazine-Genipin compounds differentially inhibit the aggregation of acetylcholinesterase (AChE) and A1–42 peptides, which means that they may be able to repair 22.3% of the neuronal damage caused by amyloid(A) peptide toxicity. In the context of vascular diseases, several research projects have been conducted that have been used as a basis to assess GNP’s safety. It also includes drug delivery systems, pharmacology, nerve regeneration, mucosal adjuvants for vaccination, ophthalmology, oncology, cartilage or bone, and food sciences.[1]

Genipin increases extracellular matrix synthesis preventing corneal perforation
Corneal melting and perforation are feared sight-threatening complications of infections, autoimmune disease, and severe burns. Assess the use of genipin in treating stromal melt. A model for corneal wound healing was created through epithelial debridement and mechanical burring to injure the corneal stromal matrix in adult mice. Murine corneas were then treated with varying concentrations of genipin, a natural occurring crosslinking agent, to investigate the effects that matrix crosslinking using genipin has in wound healing and scar formation. Genipin was used in patients with active corneal melting. RESULTS: Corneas treated with higher concentrations of genipin were found to develop denser stromal scarring in a mouse model. In human corneas, genipin promoted stromal synthesis and prevention of continuous melt. Genipin mechanisms of action create a favorable environment for upregulation of matrix synthesis and corneal scarring. These data suggest that genipin increases matrix synthesis and inhibits the activation of latent transforming growth factor-β. These findings are translated to patients with severe corneal melting.[2]
Genipin promotes the apoptosis and autophagy of neuroblastoma cells
This study investigated the underlying function and mechanism of genipin in neuroblastoma (NB). Using flow cytometry analysis and cytotoxicity tests, in vitro studies were conducted to assess the effects of genipin on the SK-N-SH cell line. The mechanism of action of genipin was explored through immunofluorescence staining, Western blotting, and caspase-3 activity assays. In addition, we also created a xenograft tumour model to investigate the effects of genipin in vivo. This research confirmed that genipin suppressed cell viability, induced apoptosis, and promoted autophagy, processes that are likely linked to the inhibition of the PI3K/AKT/mTOR signalling pathway. Autophagy inhibition increases the sensitivity of SK-N-SH cells to genipin. Furthermore, combination treatment with a PI3K inhibitor enhanced the therapeutic efficacy of genipin. These results highlight the potential of genipin as a candidate drug for the treatment of NB.[3]
Genipin Inhibits the Development of Osteosarcoma through PI3K/AKT Signaling Pathway
Osteosarcoma is a highly invasive and early metastatic tumor. At present, the toxic and side effects of chemotherapy affect the quality of life of cancer patients to varying degrees. Genipin is an extract of the natural medicine gardenia with various pharmacological activities. The purpose of this study was to investigate the effect of Genipin on osteosarcoma and its potential mechanism of action.
Crystal violet staining, MTT assay and colony formation assay were used to detect the effect of genipin on the proliferation of osteosarcoma. The effects of vitexin on migration and invasion of osteosarcoma were detected by scratch healing assay and transwell assay. Hoechst staining and flow cytometry were used to detect the effect of genipin on apoptosis of osteosarcoma cells. The expression of related proteins was detected by Western blot. An orthotopic tumorigenic animal model was used to verify the effect of genipin on osteosarcoma in vivo.
The results of crystal violet staining, MTT method and colony formation method proved that genipin significantly inhibited the proliferation of osteosarcoma cells. The results of the scratch healing assay and transwell assay showed that gen significantly inhibited the migration and invasion of osteosarcoma cells. The results of Hoechst staining and flow cytometry showed that genipin significantly promoted the apoptosis of osteosarcoma cells. The results of animal experiments show that genipin has the same anti-tumor effect in vivo. Genipin may inhibit the growth of osteosarcoma through PI3K/AKT signaling. Genipin can inhibit the growth of human osteosarcoma cells, and its mechanism may be related to the regulation of PI3K/AKT signaling pathway.[4]
Genipin-activating PPARγ impedes CCR2-mediated macrophage infiltration
A high postoperative tumour recurrence rate has significantly rendered a poorer prognosis in hepatocellular carcinoma (HCC) patients. The aim of this study is to identify a natural compound genipin as a potential and effective candidate to suppress the postoperative recurrence of HCC. Clinical analysis revealed that infiltration of macrophage into the adjacent tissue but not HCC predicted patients' poor prognosis on recurrence-free survival. Genipin intervention suppressed the Ly6C+CD11b+F4/80+ pro-inflammatory macrophage infiltration in the postoperative liver of mice. Adoptive transfer of pro-inflammatory monocytic cells completely abolished the inhibitory effect of genipin on HCC recurrence. Transcriptomic analysis on FACs-sorted macrophages from the postoperative livers of mice revealed that PPARγ signalling was involved in the regulatory effect of genipin. Genipin is directly bound to PPARγ, causing PPARγ-induced p65 degradation, which in turn suppressed the transcriptional activation of CCR2 signalling. PPARγ antagonist GW9662 abrogated the effects of genipin on CCR2-medaited macrophage infiltration as well as HCC recurrence. Cytokine array analysis identified that genipin intervention potently suppressed the secretion of CCL2 further partially contributed to the pro-inflammatory macrophage infiltration into the postoperative liver. Multiplex immunostaining on tissue array of human HCC revealed that PPARγ expression was inversely associated with CCL2 and the macrophage infiltration in the adjacent liver of HCC patients. Our works provide scientific evidence for the therapeutic potential of genipin as a PPARγ agonist in preventing postoperative recurrence of HCC.[5]
References
1. Ahmed R, Ul Ain Hira N, Wang M, Iqbal S, Yi J, Hemar Y. Genipin, a natural blue colorant precursor: Source, extraction, properties, and applications. Food Chem. 2024;434:137498. doi:10.1016/j.foodchem.2023.137498
2. Donovan C, Sun M, Cogswell D, Margo CE, Avila MY, Espana EM. Genipin increases extracellular matrix synthesis preventing corneal perforation. Ocul Surf. 2023;28:115-123. doi:10.1016/j.jtos.2023.02.003
3. Liu X, Zhou C, Cheng B, et al. Genipin promotes the apoptosis and autophagy of neuroblastoma cells by suppressing the PI3K/AKT/mTOR pathway. Sci Rep. 2024;14(1):20231. Published 2024 Aug 30. doi:10.1038/s41598-024-71123-w
4. Huang X, Jiwa H, Xu J, Zhang J, Huang Y, Luo X. Genipin Inhibits the Development of Osteosarcoma through PI3K/AKT Signaling Pathway. Curr Pharm Des. 2023;29(16):1300-1310. doi:10.2174/1381612829666230508145533
5. Wu J, Chan YT, Lu Y, et al. Genipin-activating PPARγ impedes CCR2-mediated macrophage infiltration into postoperative liver to suppress recurrence of hepatocellular carcinoma. Int J Biol Sci. 2023;19(16):5257-5274. Published 2023 Oct 16. doi:10.7150/ijbs.87327
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringCefaclor is a bactericidal antibiotic recommended for treating diverse types of infections. Its chemistry,pharmacology and pharmacokinetics were reported.....
Oct 15,2025DrugsGenipin
6902-77-8You may like
- Genipin
-
- $50.00 / 20mg
- 2025-12-05
- CAS:6902-77-8
- Min. Order:
- Purity: 99.74%
- Supply Ability: 10g
- Genipin
-

- $0.00 / 1g
- 2025-12-05
- CAS:6902-77-8
- Min. Order: 1g
- Purity: ≥98.0%
- Supply Ability: 10kg/month
- 1,4a,5,7a-Tetrahydro-1-hydroxy-7-(hydroxymethyl)-cyclopenta(c)pyran-4-carboxylic acid methyl ester
-

- 2025-12-05
- CAS:6902-77-8
- Min. Order:
- Purity: 0.99
- Supply Ability: