Practical Applications of Sodium Acetate
Nov 12,2019
Sodium acetate salt, or simply sodium acetate, has many practical uses. It is the conjugate base of a weak acid, meaning that it only partially ionizes when dissolved in water. This provides sodium acetate with buffering properties, that is the ability to maintain solutions at a relatively constant pH despite acid or base challenges. This property along with its low toxicity, helps explain why sodium acetate can be found in industries ranging from petroleum production to food flavoring.

Physical Properties
Physically, sodium acetate appears as a white hygroscopic or water-attracting crystalline powder. The pure substance has a melting point of 58 degrees C or 136 degrees F, and completely decomposes at the boiling point of 120 degrees C or 248 degrees F. Sodium acetate dissolves readily in water, having a solubility of 500 g/L at 20 degrees C. Crystals have a basic pH of about 7.5 to 9.0.
Safety
The safety of sodium acetate has been studied extensively in rat and mouse animal models. When given orally, the lethal dose that will kill half a population of rats is 3530mg of sodium acetate per kg of rat body weight. If inhaled rather than ingested, the dose required to kill half the rat population is much higher, over 30 g/m3 per hour.
In mice, a subcutaneous or under the skin injection of 3200mg/kg of body weight will kill half of a mouse population, similar to the ingestion of sodium acetate in rats. However, orally mice can withstand much more than rats; the lethal dose for half the population of mice being 6891mg/kg of body weight. In humans, inhalation of sodium acetate may cause a cough and sore throat. Direct skin or eye contact may cause redness and irritation. However, overall, toxicity in humans is minimal.
Practical Applications
Sodium acetate finds use in an extremely diverse range of industries. In the textile industry, sodium acetate neutralizes sulfuric acid waste streams and improves the wearing quality of finished fabrics. In photography, sodium acetate constitutes part of the developer solution and acts as a photo resist agent. In rubber production, sodium acetate retards vulcanization helping control the overall process.
Sodium acetate added to foods acts as a preservative, and a flavoring agent. In particular, potato chips with sodium acetate have a distinctive "salt and vinegar" taste. Sodium acetate and acetic acid solutions act as buffers to maintain relatively constant pH, a property useful both for biochemical research reactions, the petroleum industry and in the cosmetic industry. In the medical field, sodium acetate solutions treat patients with high blood acid levels and/or low sodium levels.
- Related articles
- Related Qustion
- Is Sodium acetate an acid or a base salt? Jul 16, 2024
Sodium acetate (CH3COONa) is in some cases a basic salt. CH3COONa is produced by the reaction of acetic acid with sodium hydroxide and it is the salt of the acetate ion.
- Effect of short-term feeding of sodium acetate on milk fat yield in dairy cows Dec 19, 2023
Supplementation with sodium acetate (NaAcet) increases milk fat production through an apparent stimulation of de novo lipogenesis in the mammary gland.
- Sodium Acetate: Preparation, Reactions and Applications Apr 12, 2023
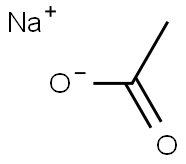
Sodium acetate, CH3COONa, also abbreviated NaOAc, is the sodium salt of acetic acid. This colorless deliquescent salt has a wide range of uses.
Tesmilifene is a small molecule chemopotentiator under development by YM BioSciences, a Candian pharmaceutical company that specialises in the development of cancer treatments.....
Nov 12,2019APIPalmitic acid is a saturated fat. It’s naturally found in some animal products like meat and dairy, as well as in palm and coconut oils. Because these two oils are frequently used in processed foods, you might be getting palmitic acid in yo....
Nov 12,2019Organic ChemistrySodium acetate
127-09-3You may like
- Sodium acetate anhydrous
-

- $10.00 / 1kg
- 2025-12-16
- CAS:127-09-3
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 100kg
- Sodium acetate
-

- $29.00 / 1g
- 2025-12-16
- CAS:127-09-3
- Min. Order:
- Purity: 98.00%
- Supply Ability: 10g
- Sodium acetate
-
- 2025-12-15
- CAS:127-09-3
- Min. Order:
- Purity: 0.99
- Supply Ability: