Quinocetone: Overview, Pharmacological Activity and Pharmacokinetics
May 24,2024
General Description
Quinocetone, a quinoxaline 1,4-dioxide derivative initially developed as an animal feed additive, has shown potential for enhancing meat production but raised concerns due to its toxicological profile. Recent studies indicate its adverse effects, including inhibition of cell viability, DNA damage, and disruption of mitochondrial function. Pharmacologically, quinocetone induces autophagy via the ER stress pathway, involving ATF6/DAPK1-mediated mAtg9a trafficking. Its pharmacokinetics in pigs and chickens involve rapid absorption and elimination, low bioavailability, and extensive metabolism yielding various metabolites. Understanding these aspects is crucial for evaluating the safety and efficacy of quinocetone in veterinary applications.

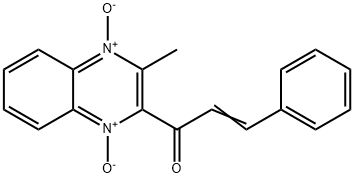
Figure 1. Quinocetone
Overview
Quinocetone, chemically known as 1-(3-methyl-2-quinoxalinyl)-3-phenyl-2propen-1-one N,N 0-dioxide, is a novel derivative of quinoxaline 1,4-dioxides (QdNOs). Initially developed by the Lanzhou Institute of Animal Husbandry and Veterinary Drugs, Chinese Academy of Agricultural Sciences, it gained attention in 2003 for its potential as an animal feed additive. Studies demonstrated its efficacy in enhancing meat production in livestock and poultry while ensuring food safety standards. However, recent research has raised concerns regarding its toxicological profile. Both in vitro and in vivo studies have highlighted potential adverse effects of quinocetone. In laboratory settings, it has been shown to inhibit cell viability, induce DNA damage, cause cell cycle arrest, trigger reactive oxygen species (ROS) production, and disrupt mitochondrial function in cell lines such as HepG2 and Vero. These findings underscore the importance of further investigating the safety implications of quinocetone, particularly in the context of animal feed additives and food production. 1
Pharmacological Activity
The pharmacological activity of quinocetone involves the induction of autophagy through the endoplasmic reticulum (ER) stress signaling pathway, as revealed by a study conducted by Yan Zhou et al. Quinocetone's mechanism of action was investigated through various experimental approaches, including Western blotting, green fluorescence protein (GFP)-LC3 vector transfection, and molecular assays. The study found that quinocetone induces autophagy in a time- and dose-dependent manner. Additionally, it was observed that quinocetone triggers ER stress, as evidenced by the upregulation of immunoglobulin heavy chain binding protein (BiP), C/EBP homologous protein (CHOP), and the transcription factors BiP, HerpUD, and sec24D. Furthermore, quinocetone led to the cleavage of ATF6, phosphorylation of MRLC (myosin regulatory light chain), and increased expression of death-associated protein kinase 1 (DAPK1). Importantly, the study highlighted the involvement of ATF6/DAPK1-mediated mAtg9a trafficking in quinocetone-induced autophagy. Quinocetone was found to stimulate MRLC-mediated mAtg9 trafficking, crucial for autophagosome formation, via the ATF6-induced upregulation of DAPK1 expression. To further confirm the role of ATF6 and DAPK1 in quinocetone-induced autophagy, stable knockdown HepG2 cell lines for ATF6 and DAPK1 were generated. Upon treatment with quinocetone, a decrease in the conversion ratios of LC3, a marker of autophagy, was observed in these cell lines. In summary, quinocetone induces autophagy through the ER stress signaling pathway-mediated cytoskeleton activation, involving the ATF6/DAPK1-modulated mAtg9a trafficking. These findings provide insights into the pharmacological activity and molecular mechanisms underlying quinocetone-induced autophagy. 1
Pharmacokinetics
Quinocetone exhibits complex pharmacokinetics characterized by rapid absorption and elimination in pigs and chickens. The absorption half-lives for pigs and chickens are approximately 0.46 hours and 0.51 hours, respectively, while the elimination half-lives are around 3.7 hours and 4.6 hours, respectively. Despite its fast absorption and excretion, the bioavailability of Quinocetone is relatively low, with values of 0.5% for pigs and 3.0% for chickens following oral administration. Quinocetone undergoes extensive metabolism prior to excretion, with multiple metabolites identified in various tissues and bodily fluids. The primary metabolic pathways include N-oxide group reduction, carbonyl reduction, alkenyl hydrogenation, oxidation, and cleavage of the carbonyl group. Notably, the N-oxide group reduction is a major pathway, particularly at position 1 due to favorable electronic effects. Studies utilizing advanced analytical techniques such as LC-MS/MS have identified numerous Quinocetone metabolites in animals. For instance, in rat liver microsomes, twenty-seven metabolites were tentatively identified, including reduction metabolites and metabolites involving hydroxylation. In swine urine, thirty-one metabolites were detected, with varying distributions across different tissues and bodily fluids. The metabolic pathways of Quinocetone in animals involve the production of various metabolites, including enantiomeric secondary alcohols resulting from carbonyl reduction. Additionally, the abundance of certain metabolites, such as 1-desoxyquinocetone, suggests preferential metabolic pathways under specific conditions. In summary, the pharmacokinetics of Quinocetone involve rapid absorption, extensive metabolism, and relatively low bioavailability. Understanding its metabolic pathways is crucial for assessing its safety and efficacy in veterinary use. 2
Reference
1. Zhou Y, Zhang S, Dai C, et al. Quinocetone triggered ER stress-induced autophagy via ATF6/DAPK1-modulated mAtg9a trafficking. Cell Biol Toxicol. 2016; 32(2): 141-152.
2. Liu ZY, Sun ZL. The metabolism of carbadox, olaquindox, mequindox, quinocetone and cyadox: an overview. Med Chem. 2013; 9(8): 1017-1027.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringAluminum oxide, with the chemical formula Al2O3, is an amphoteric oxide commonly referred to as alumina.....
May 24,2024Inorganic chemistryQuinocetone
81810-66-4You may like
- Rolapitant Synthesis
Dec 22, 2025
- Synthesis of 2-(2-Chlorophenyl)cyclohexanone
Dec 22, 2025
- Preparation methods and application of 2-(2-Ethoxyethoxy)ethyl acrylate
Dec 22, 2025
- Quinocetone
-
- $56.00 / 500mg
- 2025-12-22
- CAS:81810-66-4
- Min. Order:
- Purity: 99.77%
- Supply Ability: 10g
- Quinocetone
-

- $1.00 / 1KG
- 2025-12-11
- CAS:81810-66-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- Quinocetone
-

- $0.00 / 1kg
- 2025-06-20
- CAS:81810-66-4
- Min. Order: 1kg
- Purity: 98.00%
- Supply Ability: 20tons